Nonferrous metal, Metallic powders, Metal Compounds,Metal products from www.metal-powder-dust.com
Friday, September 28, 2012
Spherical Plain Bearings-Capability and choice of rod ends-SAZP...S on/about the!
Joint bearing according to different classification method, according to the can bear load direction or nominal contact Angle is different, divided into to the heart joint bearing and thrust joint bearing.
1, centripetal knuckle bearing - used to bear radial load, its nominal contact Angle from 0 degrees to 45 degrees, according to its nominal contact Angle is different, and can be divided into:
2. Radial contact centripetal knuckle bearing - nominal contact Angle 0 degree to the heart joint bearing, suitable for bear radial load. But also can bear radial load at the same time, bear not big axial load.
3. Angular contact centripetal knuckle bearing - nominal contact Angle is greater than 0 degrees, but less than or equal to 45 degrees to the heart joint bearing, can bear radial load and axial load at the same time, the role of joint load.
More about:Spherical Plain Bearings-Capability and choice of rod ends-SAZP...S suppliers
Read more:Shaft Bearing material
Wednesday, September 26, 2012
Spherical Plain Bearings - Capability and choice of rod ends - SA... The characteristics of C!
Spherical Plain Bearings - Capability and choice of rod ends - SA... C material is carbon steel, galvanized, no lubrication, high capacity, self-lubricating rod end components from the body and self-lubricating rod end bearing GE heart... C or GE... , easy to install and disassemble and institutions, and the advantages of simplified.
More about:Spherical Plain Bearings-Capability and choice of rod ends-SA...C suppliers
Read more:Shaft Bearing material
Sunday, September 23, 2012
Spherical Plain Bearings - Capability and choice of rod ends - SK... ES simple features and application!
Spherical Plain Bearings - Capability and choice of rod ends - SK... ES, rod body material is carbon steel, the surface phosphating, rod end handle cotter pin for welding position, hole oil lubrication cup or rod end, carrying capacity is big, easy to install and remove the advantage simplified organization.
More about:Spherical Plain Bearings-Capability and choice of rod ends-SK...ES price
Read more:Shaft Bearing material
Thursday, September 20, 2012
Spherical Plain Bearings - Capability and choice of rod ends - SK... ES introduced!
Spherical Plain Bearings - Capability and choice of rod ends - SK... ES rod body material is carbon steel, the surface phosphating, rod end handle cotter pin for welding position, hole oil lubrication cup or rod end, carrying capacity is big, easy to install and remove the advantage simplified organization.
More about:buy Spherical Plain Bearings-Capability and choice of rod ends-SK...ES
Read more:Shaft Bearing material
Tuesday, September 18, 2012
Spherical Plain Bearings - Maintenance - free radial Spherical Plain Bearings - GE... C characteristics and purpose!
Spherical Plain Bearings - Maintenance - free radial Spherical Plain Bearings - GE... C, outer material is carbon steel, extrusion, PTFE lining spherical composite material, internal material is bearing steel, quenching, hard chromium plating sphere. Self lubrication, resistance to mo losses, self-aligning, low friction characteristics, suitable for low speed and heavy machinery industry.
More about: Spherical Plain Bearings-Maintenance-free radial spherical plain bearings-GE...C suppliers
Read more: Shaft Bearing material
Sunday, September 16, 2012
Spherical Plain Bearings - Maintenance - free radial Spherical Plain Bearings - GEG... XT - 2 rs related introduced!
Resistance ring inside and larger diameter, bearing arrangement need a greater Angle, suitable for large load mechanism. Outer fracture of high carbon chromium steel, hardening and phosphating, and on both sides of the lip seal, has the sliding surface of the PTFE fabric. Outside of carbon, inner ring around the press and polytetrafluoroethylene (PTFE) composites sliding surface.
More about: Spherical Plain Bearings-Maintenance-free radial spherical plain bearings-GEG...XT-2RS price
Read more:Shaft Bearing material
Friday, September 14, 2012
Spherical Plain Bearings - Maintenance - free radial Spherical Plain Bearings - GEZ... ET - 2 rs description!
Spherical Plain Bearings - Maintenance - free radial Spherical Plain Bearings - GEZ... ET - 2RS rsouter ring of carbon chromium steel, fractured, hardened and phosphated, With two seals at boths sides, With sliding surface of PTFE fabric; Inner ring of carbon chromium steel, hardened, Sliding surface treated with hard chromium plating. The Outer ring of the XT design axially split twice, held together by reltaining rings.
More about: Spherical Plain Bearings-Maintenance-free radial spherical plain bearings-GEZ...ET-2RS
Read more: Shaft Bearing material
Tuesday, September 11, 2012
Spherical Plain Bearings-Maintenance-free radial spherical plain bearings-GEH... HT patent what effect is there?
Spherical Plain Bearings-Maintenance-free radial spherical plain bearings-GEH... HT patent,Study in terms of its role should be to support, that is used for bearing the literal interpretation, but this is only a part of its roles, supporting its essence is able to bear the radial load. Can also be understood as it is used to a fixed axis. Is a fixed axis so that it can only be achieved by turning, and the control of its axial and radial movement. Motor without bearing the consequences is simply not work. Because the shaft may move in any direction, and electrical work required rotate shaft only.
Theoretically impossible transmission effect, not only such, bearing will affect the transmission, in order to reduce the impact on the high speed shaft bearing must achieve good lubrication, some bearing itself has lubrication, called pre lubricating bearings, while most of the Zhou Chengbi must have oil, load at high speed operation, due to friction not only will increase energy consumption, more terrible is easily damaged bearing. The sliding friction into rolling friction is one-sided, because there is called sliding bearing.
More about: Spherical Plain Bearings-Maintenance-free radial spherical plain bearings-GEH...HT patent suppliers
Read more:Shaft Bearing material
Friday, September 7, 2012
What is Spherical Plain Bearings - Radial Spherical Plain Bearings requiring maintenance - GE... XS/K?
Bearing is in mechanical transmission process up fixed and reduce load coefficient of friction components. Can say, when other parts in the shaft to move relative to each other, used to reduce power transfer process of friction coefficient and keep the axle center of the fixed part. Bearing is a kind of very important contemporary machinery parts. Its main function is to support mechanical rotator, in order to reduce the equipment in the transmission process of mechanical load coefficient of friction. According to the motion element frictional qualitative different, bearing can be divided into the rolling bearing and sliding bearing two kinds.
More about: Spherical Plain Bearings-Radial spherical plain bearings requiring maintenance-GE...XS/K suppliers
Read more: Shaft Bearing material
Wednesday, September 5, 2012
Spherical Plain Bearings - Radial Spherical Plain Bearings requiring maintenance - GEGZ... ES description!
Spherical Plain Bearings - Radial Spherical Plain Bearings requiring maintenance - GEGZ... ES description is as follows:
TOT centripetal spherical sliding bearing need maintenance (steel on steel) in two ring on the sliding contact surface hardening. Surface treatment and molybdenum disuphide and phosphate. It has characterisdics wear resistance and wear corrosion. The sliding contact surface combined bearing requires regular and lubrication. The sliding contact surface of high intensity, make these bearing, especially suitable for alternating direction, impact load or heavy static load to accommodate the weight of the bearing arrangement.
More about: Spherical Plain Bearings-Radial spherical plain bearings requiring maintenance-GEGZ…ES price
Read more:Shaft Bearing material
Sunday, September 2, 2012
Friday, August 31, 2012
Oxycarbonyruodium chloride have what attribute
Oxycarbonyruodium chloride is Purity: Ru or 39.5% CAS number: 22941-53-3 molecular formula: 6 co · Cl4Ru2 molecular weight: 512.012 pale yellow powder, and the air is easy decomposition, soluble in alcohol, acetone and water. Ruthenium (Ru) content: Ru or 10.5% iron determine if flexion-extension (Fe) radiographs content: 0.001% or less nitrate (NO3-1) content: acuities 0.005% cold dry sealed storage.
More about: Oxycarbonyruodium chloride sales
Read more: Chemical products
More about: Oxycarbonyruodium chloride sales
Read more: Chemical products
Thursday, June 28, 2012
Where to find Welding rods for mild steel CHE557GX?
Welding is the action of abutting metals by melting the locations and again application a accompaniment to anatomy a joint. Adjustment can be done application altered activity sources, from a gas blaze or electric arc to a laser or ultrasound.
Electric adjustment is the action of heating and adjustment two pieces of metal calm application a able electric current. It was invented by assistant Elihu Thomson. It requires the use of a specialized accessory alleged a agent that releses the accepted acclimated for welding.
Unlike added acceptable methods, electric adjustment requires alone a basal bulk of accomplishment and compassionate on the allotment of the agent operator. He have to alone apprentice the able adjustment calefaction of the metal getting used, but is not appropriate to apprentice the added intricate processes of accepted welding. The agent use in electric adjustment is self-regulating, and alone needs casual lubrication to abide alive properly. This makes electric adjustment absolute for the amateur welder.
The a lot of accepted gas welding action is oxyfuel welding, aswell accepted as oxyacetylene welding. It is one of the oldest and a lot of able adjustment processes, but in contempo years it has become beneath accepted in automated applications. It is still broadly acclimated for adjustment pipes and tubes, as able-bodied as adjustment work.
The accessories is almost bargain and simple, about employing the agitation of acetylene in oxygen to aftermath a adjustment blaze temperature of about 3100 °C. The flame, back it is beneath concentrated than an electric arc, causes slower bond cooling, which can advance to greater balance stresses and bond distortion, admitting it eases the adjustment of top admixture steels. A agnate process, about alleged oxyfuel cutting, is acclimated to cut metals
More about: Welding rods for mild steel CHE557GX sale
Read more: welding products
Monday, June 25, 2012
What is Anti-DBH (Dopamine-β-Hydroxylase)?
Dopamine β-hydroxylase (DBH) is an agitator that converts dopamine to norepinephrine.
DBH is a 290 kDa copper-containing oxygenase consisting of four identical subunits, and its action requires ascorbate as a cofactor. It is the alone agitator complex in the amalgam of small-molecule neurotransmitters that is membrane-bound, authoritative norepinephrine the alone transmitter actinic axial vesicles. It is bidding in noradrenergic assumption terminals of the axial and borderline afraid systems, as able-bodied as in chromaffin beef of the adrenal medulla. DBH is inhibited by disulfiram, tropolone, and, a lot of selectively, by nepicastat.
Dopamine beta hydroxylase absence is a action involving bare Dopamine beta hydroxylase.
A absence of norepinephrine and epinephrine that causes orthostatic hypotension, nasal stuffiness, and bent eyelids (ptosis) a part of added symptoms. Some humans accredit to this as norepinephrine deficiency. It's acquired by abundantly added amounts of serum dopamine and absolution of dopamine in abode of norepinephrine. It is difficult for humans with this to angle still for best than one minute. Another evidence is hypoglycemia which is anticipation to be acquired by adrenomedullary abortion and the T-wave abnormalities from abortion of noradrenergic control. Also, prolactin is frequently suppressed by boundless dopamine. This can aswell accept an appulse on digestion. "Dopamine has an emetic aftereffect and inhibitis digestive motricity". Humans with dopamine beta hydroxylase absence may accept a abstruse acknowledgment to NRI antidepressant medication such as Edronax -- they may acquaintance added depression, beneath energy, and become added sleepy. This is a anatomy of dysautonomia but differentiated from familial dysautonomia by abridgement of familial dysautonomic affection such as accident of faculty of affliction and smell.
More about: Anti-DBH (Dopamine-β-Hydroxylase) sale
Read more: Reagent Product
Thursday, June 21, 2012
Where to get Welding consumables for marine pojects CHE50?
Welding is the action of abutting metals by melting the locations and again application a accompaniment to anatomy a joint. Adjustment can be done application altered activity sources, from a gas blaze or electric arc to a laser or ultrasound.
Welding is a artifact or sculptural action that joins materials, usually metals or thermoplastics, by causing coalescence. This is generally done by melting the workpieces and abacus a accompaniment actual to anatomy a basin of aqueous actual (the band pool) that cools to become a able joint, with burden sometimes acclimated in affiliation with heat, or by itself, to aftermath the weld. This is in adverse with soldering and brazing, which absorb melting a lower-melting-point actual amid the workpieces to anatomy a band amid them, after melting the workpieces.
Many altered activity sources can be acclimated for welding, including a gas flame, an electric arc, a laser, an electron beam, friction, and ultrasound. While generally an automated process, adjustment may be performed in abounding altered environments, including accessible air, beneath baptize and in alien space. Adjustment is a potentially chancy adventure and precautions are appropriate to abstain burns, electric shock, eyes damage, assimilation of poisonous gases and fumes, and acknowledgment to acute ultraviolet radiation.
A consumable is, according to the 1913 copy of Webster's Dictionary, something that is able of getting consumed; that may be destroyed, dissipated, wasted, or spent. John Locke specifies these as "consumable commodities."
Consumables are articles that consumers buy recurrently, i.e., items which "get acclimated up" or discarded.
For archetype accessible appointment food are such articles as paper, pens, book folders, post-it notes, computer disks, and toner or ink cartridges. Not included basic appurtenances such as computers, fax machines, and added business machines or appointment furniture.
For arc adjustment one uses a accessible electrode. This is an electrode that conducts electricity to the arc but aswell melts into the band as a accompaniment metal.
More about: Welding consumables for marine pojects CHE50 sale
Read more: welding products
Monday, June 18, 2012
Characteristics of Anti-CXC-R3/CD183 (Chemokine receptorC-X-C chemokine receptor type 3)
CXC chemokine receptors are basic film proteins that accurately bind and acknowledge to cytokines of the CXC chemokine family. They represent one subfamily of chemokine receptors, a ample ancestors of G protein-linked receptors that are accepted as seven transmembrane (7-TM) proteins, back they amount the corpuscle film seven times. There are currently seven accepted CXC chemokine receptors in mammals, alleged CXCR1 through CXCR7.
CXCR3 is bidding predominantly on T lymphocytes, and aswell on added lymphocytes (some B beef and NK cells) and is awful induced afterward corpuscle activation. There are two isoforms, CXCR3-A and CXCR3-B. It has three awful accompanying ligands in mammals, CXCL9, CXCL10 and CXCL11.
Chemokine receptors are cytokine receptors begin on the apparent of assertive beef that collaborate with a blazon of cytokine alleged a chemokine. There accept been 19 audible chemokine receptors declared in mammals. Each has a 7-transmembrane (7TM) anatomy and couples to G-protein for arresting transduction aural a cell, authoritative them associates of a ample protein ancestors of G protein-coupled receptors. Afterward alternation with their specific chemokine ligands, chemokine receptors activate a alteration in intracellular calcium (Ca2+) ions (calcium signaling). This causes corpuscle responses, including the access of a action accepted as chemotaxis that traffics the corpuscle to a adapted area aural the organism. Chemokine receptors are disconnected into altered families, CXC chemokine receptors, CC chemokine receptors, CX3C chemokine receptors and XC chemokine receptors that accord to the 4 audible subfamilies of chemokines they bind.
Characteristics
Chemokine receptors are G protein-coupled receptors absolute 7 transmembrane domains that are begin predominantly on the apparent of leukocytes. Approximately 19 altered chemokine receptors accept been characterized to date, which allotment abounding accepted structural features; they are composed of about 350 amino acids that are disconnected into a abbreviate and acerb N-terminal end, seven circling transmembrane domains with three intracellular and three extracellular hydrophilic loops, and an intracellular C-terminus absolute serine and threonine residues that act as phosphorylation sites during receptor regulation. The aboriginal two extracellular loops of chemokine receptors are affiliated calm by disulfide bonding amid two conserved cysteine residues. The N-terminal end of a chemokine receptor binds to chemokine(s) and is important for ligand specificity. G-proteins brace to the C-terminal end, which is important for receptor signaling afterward ligand binding. Although chemokine receptors allotment top amino acerbic character in their primary sequences, they about bind a bound amount of ligands.
More about: Anti-CXC-R3/CD183 (Chemokine receptorC-X-C chemokine receptor type 3) sale
Read more: Assay kits
Thursday, June 14, 2012
How to get CHE42?
CHE42 belongs to titania calcium type.The blazon of adjustment accepted is AC-DC.Equivalent barometer is JIS D4303 .Mainly for adjustment steels A, B, E for ships or low admixture steels with agnate grade.
Calcium titanate, aswell accepted as calcium titanium oxide, is a asleep admixture with the actinic blueprint CaTiO3. As a mineral, it is alleged perovskite, called afterwards Russian mineralogist, L. A. Perovski (1792-1856). It is a colourless, diamagnetic solid, although the mineral is generally coloured attributable to impurities.
CaTiO3 can be able by the aggregate of CaO and TiO2 at temperatures >1300 °C. Sol-gel processes has been acclimated to accomplish a added authentic substance, as able-bodied as blurred the amalgam temperature. These compounds actinic are added compressible due to the powders from the sol-gel action as able-bodied and accompany it afterpiece to its affected body (~4.04 g/ml).
Calcium titanate is acquired as orthorhombic crystals, added accurately perovskite structure. In this motif, the Ti(IV) centers are octahedral and the Ca2+ centers absorb a cage of 12 oxygen centres. Many advantageous abstracts accept accompanying structures, e.g. barium titanate or variations of the structure, e.g. yttrium barium chestnut oxide.
More about: CHE42 sale
Read more: welding products
Monday, June 11, 2012
Where to get Pigment powders?
Pigment is a actual that changes the blush of reflected or transmitted ablaze as the aftereffect of wavelength-selective absorption. This concrete action differs from fluorescence, phosphorescence, and added forms of luminescence, in which a actual emits light.
Pure pigments reflect ablaze in a actual specific way that cannot be absolutely bifold by the detached ablaze emitters in a computer display. However, by authoritative accurate abstracts of pigments, abutting approximations can be made. The Munsell Blush Arrangement provides a acceptable conceptual account of what is missing. Munsell devised a arrangement that provides an cold admeasurement of blush in three dimensions: hue, amount (or lightness), and chroma. Computer displays in accepted are clumsy to appearance the accurate blush of abounding pigments, but the hue and animation can be reproduced with about accuracy. However, if the gamma of a computer affectation deviates from the advertence value, the hue is aswell systematically biased.
In biology, a colorant is any black actual of bulb or beastly cells. Abounding biological structures, such as skin, eyes, fur and hair accommodate pigments (such as melanin). Beastly derma blush is generally accomplished with specialized beef alleged chromatophores, which in animals such as the octopus and chameleon can be controlled to alter the animal's color. Abounding altitude affect the levels or attributes of pigments in plant, animal, some protista, or bane cells. For instance, Albinism is a ataxia affecting the akin of melanin assembly in animals.
Pigmentation is acclimated in bacilli for abounding biological purposes including Camouflage, Mimicry, Aposematism (warning), Sexual alternative and added forms of Signalling, Photosynthesis (in plants), as able-bodied as basal concrete purposes such as aegis from Sunburn.
Pigment blush differs from structural blush in that it is the aforementioned for all examination angles, admitting structural blush is the aftereffect of careful absorption or iridescence, usually because of multilayer structures. For example, butterfly wings about accommodate structural color, although abounding collywobbles accept beef that accommodate colorant as well.
More about: Pigment powders sale
Read more: Pigment Additives
Thursday, June 7, 2012
What is Welding Rods for Pressure Vessels CHL707R?
Welding is a artifact or sculptural action that joins materials, usually metals or thermoplastics, by causing coalescence. This is about done by melting the workpieces and abacus a accompaniment actual to anatomy a basin of aqueous actual (the bond pool) that cools to become a able joint, with burden sometimes acclimated in affiliation with heat, or by itself, to aftermath the weld.
The action is able and can be performed with almost bargain equipment, authoritative it able-bodied ill-fitted to boutique jobs and acreage work. An abettor can become analytic able with a bashful bulk of training and can accomplish ability with experience. Bond times are rather slow, back the accessible electrodes accept to be frequently replaced and because slag, the balance from the flux, accept to be chipped abroad afterwards welding. Furthermore, the action is about bound to adjustment adamant materials, admitting appropriate electrodes accept fabricated accessible the adjustment of casting iron, nickel, aluminum, copper, and added metals.
Gas metal arc adjustment (GMAW), aswell accepted as metal apathetic gas or MIG welding, is a semi-automatic or automated action that uses a connected wire augment as an electrode and an apathetic or semi-inert gas admixture to assure the bond from contamination. Back the electrode is continuous, adjustment speeds are greater for GMAW than for SMAW.
A accompanying process, flux-cored arc adjustment (FCAW), uses agnate accessories but uses wire consisting of a animate electrode surrounding a crumb ample material. This cored wire is added big-ticket than the accepted solid wire and can accomplish effluvium and/or slag, but it permits even college adjustment acceleration and greater metal penetration.
Gas tungsten arc adjustment (GTAW), or tungsten apathetic gas (TIG) welding, is a chiral adjustment action that uses a nonconsumable tungsten electrode, an apathetic or semi-inert gas mixture, and a abstracted accompaniment material. Especially advantageous for adjustment attenuate materials, this adjustment is characterized by a abiding arc and top superior welds, but it requires cogent abettor accomplishment and can alone be able at almost low speeds
More about: Welding Rods for Pressure Vessels CHL707R sale
Read more: welding products
What is Iron oxide red used for?
Iron oxide is a actinic admixture that is fabricated of oxygen and iron. Adamant is a brownish aspect that is begin in about 5% of the Earth's crust. When adamant oxidizes, or rusts, colors such as yellow, orange, and red are created. A ample bulk of adamant oxide appears on the planet Mars, which is accepted as the "Red Planet." Mars appears to be red because its band is composed mostly of adamant oxide.
This admixture is frequently accepted as hematite. Iron oxide is anticipation that adamant oxide deposits were created by the precipitation of adamant from sea baptize during the Proterozoic Eon, some 1.6 billion years ago. These deposits are begin in locations about the world, with the greatest concentrations getting in the USA, India, Australia, China, Brazil, and Russia.
In the art world, adamant oxide is acclimated to actualize pigments such as burnt sienna and burnt umber. This adjustment of creating pigments in acrylic colors has been acclimated back the aged age. The cavern paintings at Lascaux are an archetype of how continued this admixture has been acclimated in the conception of art.
The cosmetics industry uses adamant oxide to actualize assorted pigments in make-up. Because it is non-toxic, baptize repellent, and it does not run or bleed, it is an ideal accretion to cosmetics such as mascara, foundation, and eye shadow. There are two altered types of compounds acclimated in the corrective industry: adamant oxide II which has a atramentous pigment, and adamant oxide III, which is red.
More about: Iron oxide red sale
Read more: Pigment powders
Monday, June 4, 2012
Applications of Chromium(III) oxide
Chromium(III) oxide is the asleep admixture of the blueprint Cr2O3. It is one of arch oxides of chromium and is acclimated as a pigment. In nature, it occurs as the attenuate mineral eskolaite.
Properties
Cr2O3 adopts the corundum structure, consisting of a hexagonal abutting arranged arrangement of oxide anions with 2/3 of the octahedral holes active by chromium. Similar to corundum, Cr2O3 is a hard, breakable actual (Mohs acerbity 8-8.5). It is antiferromagnetic up to 307 K, the Neel temperature. It is not readily attacked by acids or bases, although aqueous acrid gives chromites (salts with the Cr2O2−4 anion, not to be abashed with the accompanying mineral chromite). It turns amber if heated, but reverts to its aphotic blooming blush if cooled. It is aswell hygroscopic.
Applications
Because of its ample stability, Chromium(III) oxide is a frequently acclimated colorant and was originally alleged viridian. It is acclimated in paints, inks, and glasses. It is the colourant in "chrome green" and "institutional green." Chromium(III) oxide is the forerunner to the alluring colorant chromium dioxide.
It is one of the abstracts that are acclimated if cutting the edges of knives on a section of covering (also alleged stropping). In this ambience it is generally accepted as blooming compound.
More about: Chromium(III) oxide sale
Read more: Pigment Additives
Thursday, May 31, 2012
How to get Welding Rods for Pressure Vessels CHS137R?
Welding is a artifact or sculptural action that joins materials, usually metals or thermoplastics, by causing coalescence. This is generally done by melting the workpieces and abacus a accompaniment actual to anatomy a basin of aqueous actual (the bond pool) that cools to become a able joint, with burden sometimes acclimated in affiliation with heat, or by itself, to aftermath the weld.
Energy axle adjustment methods, namely laser axle adjustment and electron axle welding, are almost new processes that accept become absolutely accepted in top assembly applications. The two processes are absolutely similar, differing a lot of conspicuously in their antecedent of power. Laser axle adjustment employs a awful focused laser beam, while electron axle adjustment is done in a exhaustion and uses an electron beam. Both accept a actual top activity density, authoritative abysmal bond assimilation accessible and aspersing the admeasurement of the bond area. Both processes are acutely fast, and are calmly automated, authoritative them awful productive. The primary disadvantages are their actual top accessories costs (though these are decreasing) and a susceptibility to thermal cracking. Developments in this breadth cover laser-hybrid welding, which uses attempt from both laser axle adjustment and arc adjustment for even bigger bond properties, and X-ray welding.
Welds can be geometrically able in abounding altered ways. The 5 basal types of bond joints are the abject joint, lap joint, bend joint, bend joint, and T-joint (a alternative of this endure is the cruciform joint). Other variations abide as well—for example, double-V alertness joints are characterized by the two pieces of actual anniversary cone-shaped to a individual centermost point at one-half their height. Single-U and double-U alertness joints are aswell adequately common—instead of accepting beeline edges like the single-V and double-V alertness joints, they are curved, basic the appearance of a U. Lap joints are aswell frequently added than two pieces thick—depending on the action acclimated and the array of the material, abounding pieces can be anchored calm in a lap collective geometry.
Many adjustment processes crave the use of a accurate collective designs; for example, attrition atom welding, laser axle welding, and electron axle adjustment are a lot of frequently performed on lap joints. Other adjustment methods, like cloistral metal arc welding, are acutely able and can bond about any blazon of joint. Some processes can aswell be acclimated to accomplish multipass welds, in which one bond is accustomed to cool, and again addition bond is performed on top of it. This allows for the adjustment of blubbery sections abiding in a single-V alertness joint, for example.
Many audible factors access the backbone of welds and the actual about them, including the adjustment method, the bulk and absorption of activity input, the weldability of the abject material, accompaniment material, and alteration material, the architecture of the joint, and the interactions amid all these factors. To assay the superior of a weld, either annihilative or nondestructive testing methods are frequently acclimated to verify that welds are chargeless of defects, accept adequate levels of balance stresses and distortion, and accept adequate heat-affected area (HAZ) properties. Types of adjustment defects cover cracks, distortion, gas inclusions (porosity), non-metallic inclusions, abridgement of fusion, abridged penetration, lamellar tearing, and undercutting. Adjustment codes and blueprint abide to adviser welders in able adjustment address and in how to adjudicator the superior of welds. Methods such as beheld inspection, radiography, accelerated testing, dye biting inspection, Magnetic-particle assay or automated CT scanning can advice with apprehension and assay of assertive defects.
More about: Welding Rods for Pressure Vessels CHS137R sale
Read more: Metal products
Monday, May 28, 2012
How to get Iron oxide brown?
Iron oxides are actinic compounds composed of adamant and oxygen. All together, there are sixteen accepted adamant oxides and oxyhydroxides.
Iron oxides and oxide-hydroxides are boundless in nature, play an important role in abounding geological and biological processes, and are broadly activated by humans, e.g., as adamant ores, pigments, catalysts, in thermite (see the diagram). Common blight is a anatomy of iron(III) oxide. Adamant oxides are broadly acclimated as inexpensive, abiding pigments in paints, coatings and black concretes. Colors frequently accessible are in the "earthy" end of the yellow/orange/red/brown/black range.
Iron oxide is a actinic admixture that is fabricated of oxygen and iron. Adamant is a brownish aspect that is begin in about 5% of the Earth's crust. When adamant oxidizes, or rusts, colors such as yellow, orange, and red are created. A ample bulk of adamant oxide appears on the planet Mars, which is accepted as the "Red Planet." Mars appears to be red because its band is composed mostly of adamant oxide.
This admixture is frequently accepted as hematite. It is anticipation that adamant oxide deposits were created by the precipitation of adamant from sea baptize during the Proterozoic Eon, some 1.6 billion years ago. These deposits are begin in locations about the world, with the greatest concentrations getting in the USA, India, Australia, China, Brazil, and Russia.
There are three elements that are by itself magnetic: cobalt, nickel, and iron. Among those three, adamant is the a lot of magnetic, which is why adamant oxide is acclimated abundantly in the assembly of magnets, cyberbanking parts, audio and video cassette tapes, and ATM cards.
In the art world, adamant oxide is acclimated to actualize pigments such as burnt sienna and burnt umber. This adjustment of creating pigments in acrylic colors has been acclimated back the aged age. The cavern paintings at Lascaux are an archetype of how continued this admixture has been acclimated in the conception of art.
More about: Iron oxide brown sale
Read more: Pigment Additives
Thursday, May 24, 2012
Applications of Palladium
Palladium is a actinic aspect with the actinic attribute Pd and an diminutive amount of 46. It is a attenuate and bright silvery-white metal apparent in 1803 by William Hyde Wollaston. He alleged it afterwards the asteroid Pallas, which was itself alleged afterwards the appellation of the Greek goddess Athena, acquired by her if she bulk Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium anatomy a accumulation of elements referred to as the platinum accumulation metals (PGMs). These accept agnate actinic properties, but aegis has the everyman melting point and is the atomic close of them.
The different backdrop of aegis and added platinum accumulation metals (PGMs) annual for their boundless use. A division of all appurtenances bogus today either accommodate PGMs or accept a cogent allotment in their accomplishment action played by PGMs. Over bisected of the accumulation of aegis and its congener platinum goes into catalytic converters, which catechumen up to 90% of adverse gases from auto bankrupt (hydrocarbons, carbon monoxide, and nitrogen dioxide) into less-harmful substances (nitrogen, carbon dioxide and baptize vapor). Aegis is aswell acclimated in electronics, dentistry, medicine, hydrogen purification, actinic applications, and groundwater treatment. Aegis plays a key role in the technology acclimated for ammunition cells, which amalgamate hydrogen and oxygen to aftermath electricity, heat, and water.
Applications
The better use of aegis today is in catalytic converters. Aegis is aswell acclimated in jewelry, in dentistry, watch making, in claret amoroso analysis strips, in aircraft atom plugs and in the assembly of surgical instruments and electrical contacts. Aegis is aswell acclimated to accomplish able axle flutes. As a commodity, aegis banknote has ISO bill codes of XPD and 964. Aegis is one of alone four metals to accept such codes, the others getting gold, argent and Palladium .
When it is cautiously divided, such as in aegis on carbon, aegis forms a able agitator and speeds up hydrogenation and dehydrogenation reactions, as able-bodied as in petroleum cracking. A ample amount of carbon-carbon band basic reactions in amoebic allure (such as the Heck and Suzuki coupling) are facilitated by catalysis with aegis compounds. (see #Compounds and palladium-catalyzed coupling reactions) In addition, palladium, if broadcast on conductive materials, proves to be an accomplished electrocatalyst for blaze of primary alcohols in acrid media. In 2010, palladium-catalysed amoebic reactions were recognised by the Nobel Prize in Chemistry. Aegis is aswell a able metal for constant catalysis. It is acclimated in aggregate with a ample array of ligands for awful careful actinic transformations. A 2008 abstraction showed that aegis is an able agitator for authoritative carbon-fluoride bonds. Aegis is begin in the Lindlar catalyst, aswell alleged Lindlar's Palladium.
The second-biggest appliance of aegis in electronics is in the accomplish of multilayer bowl capacitors, in which aegis (and palladium-silver alloys) are acclimated as electrodes. Aegis (sometimes adulterated with nickel) is acclimated in adapter platings in customer electronics.
It is aswell acclimated in plating of cyberbanking apparatus and in soldering materials. The cyberbanking area captivated 1.07 actor troy ounces (33.2 tonnes) of aegis in 2006, according to a Johnson Matthey report.
Hydrogen calmly diffuses through acrimonious palladium; thus, it provides a agency of antibacterial the gas. Membrane reactors with Pd membranes are accordingly acclimated for the assembly of top abstention hydrogen. Aegis is a allotment of the palladium-hydrogen electrode in electrochemical studies. Palladium(II) chloride can burn ample amounts of carbon monoxide gas, and is acclimated in carbon monoxide detectors.
More about: Palladium sale
Read more: Metal products
Wednesday, April 25, 2012
What is Iron oxide blue used for?
Iron oxide is a actinic admixture that is fabricated of oxygen and iron. Adamant is a brownish aspect that is begin in about 5% of the Earth's crust. When adamant oxidizes, or rusts, colors such as yellow, orange, and red are created. A ample bulk of adamant oxide appears on the planet Mars, which is accepted as the "Red Planet." Mars appears to be red because its band is composed mostly of adamant oxide.
This admixture is frequently accepted as hematite. It is anticipation that adamant oxide deposits were created by the precipitation of adamant from sea baptize during the Proterozoic Eon, some 1.6 billion years ago. These deposits are begin in locations about the world, with the greatest concentrations getting in the USA, India, Australia, China, Brazil, and Russia.
There are three elements that are by itself magnetic: cobalt, nickel, and iron. Among those three, adamant is the a lot of magnetic, which is why adamant oxide is acclimated abundantly in the assembly of magnets, cyberbanking parts, audio and video cassette tapes, and ATM cards.
In the art world, Iron oxide is acclimated to actualize pigments such as burnt sienna and burnt umber. This adjustment of creating pigments in acrylic colors has been acclimated back the aged age. The cavern paintings at Lascaux are an archetype of how continued this admixture has been acclimated in the conception of art.
The cosmetics industry uses adamant oxide to actualize assorted pigments in make-up. Because it is non-toxic, baptize repellent, and it does not run or bleed, it is an ideal accretion to cosmetics such as mascara, foundation, and eye shadow. There are two altered types of compounds acclimated in the corrective industry: adamant oxide II which has a atramentous pigment, and adamant oxide III, which is red.
The altered pigments of this admixture are acclimated to dye such things as paint, concrete, leather, shoe polish, tiles, and rubber. Amber pigments can ambit in blush from a ablaze amber to a coffee blush brown. Red pigmentations ambit in blush from a abysmal orange to a abysmal red.
Iron oxide is aswell added to altered nutrients, feeds, and medications. It can be begin in assertive types of bloom products, such as talcum powder, facial cream, and physique cream. Some sunblock articles accommodate adamant oxide, which helps block UV application from damaging animal skin.
More about: Iron oxide blue sale
Read more: Pigment powders
This admixture is frequently accepted as hematite. It is anticipation that adamant oxide deposits were created by the precipitation of adamant from sea baptize during the Proterozoic Eon, some 1.6 billion years ago. These deposits are begin in locations about the world, with the greatest concentrations getting in the USA, India, Australia, China, Brazil, and Russia.
There are three elements that are by itself magnetic: cobalt, nickel, and iron. Among those three, adamant is the a lot of magnetic, which is why adamant oxide is acclimated abundantly in the assembly of magnets, cyberbanking parts, audio and video cassette tapes, and ATM cards.
In the art world, Iron oxide is acclimated to actualize pigments such as burnt sienna and burnt umber. This adjustment of creating pigments in acrylic colors has been acclimated back the aged age. The cavern paintings at Lascaux are an archetype of how continued this admixture has been acclimated in the conception of art.
The cosmetics industry uses adamant oxide to actualize assorted pigments in make-up. Because it is non-toxic, baptize repellent, and it does not run or bleed, it is an ideal accretion to cosmetics such as mascara, foundation, and eye shadow. There are two altered types of compounds acclimated in the corrective industry: adamant oxide II which has a atramentous pigment, and adamant oxide III, which is red.
The altered pigments of this admixture are acclimated to dye such things as paint, concrete, leather, shoe polish, tiles, and rubber. Amber pigments can ambit in blush from a ablaze amber to a coffee blush brown. Red pigmentations ambit in blush from a abysmal orange to a abysmal red.
Iron oxide is aswell added to altered nutrients, feeds, and medications. It can be begin in assertive types of bloom products, such as talcum powder, facial cream, and physique cream. Some sunblock articles accommodate adamant oxide, which helps block UV application from damaging animal skin.
More about: Iron oxide blue sale
Read more: Pigment powders
Monday, April 23, 2012
Applications of Titanium dioxide, rutile
Titanium dioxide, aswell accepted as titanium(IV) oxide or titania, is the by itself occurring oxide of titanium, actinic blueprint TiO2. If acclimated as a pigment, it is alleged titanium white, Colorant White 6, or CI 77891. Generally it comes in two altered forms, rutile and anatase. It has a advanced ambit of applications, from acrylic to sunscreen to aliment colouring. If acclimated as a aliment colouring, it has E amount E171.
Applications
Titanium dioxide is the a lot of broadly acclimated white colorant because of its accuracy and actual top refractive index, in which it is surpassed alone by a few added materials. Approximately 4 actor bags of pigmentary TiO2 are captivated annually worldwide. If deposited as a attenuate film, its refractive basis and colour accomplish it an accomplished cogitating optical blanket for dielectric mirrors and some gemstones like "mystic blaze topaz". TiO2 is aswell an able opacifier in crumb form, area it is active as a colorant to accommodate whiteness and caliginosity to articles such as paints, coatings, plastics, papers, inks, foods, medicines (i.e. pills and tablets) as able-bodied as a lot of toothpastes. In paint, it is generally referred to offhandedly as "the absolute white", "the whitest white", or added agnate terms. Caliginosity is bigger by optimal allocation of the titanium dioxide particles.
In corrective and derma affliction products, titanium dioxide is acclimated as a pigment, sunscreen and a thickener. It is aswell acclimated as a boom colorant and in styptic pencils. Titanium dioxide is produced in capricious atom sizes, oil and baptize dispersible, and with capricious coatings for the corrective industry. This colorant is acclimated abundantly in plastics and added applications for its UV aggressive backdrop area it acts as a UV absorber, calmly transforming annihilative UV ablaze activity into heat.
Titanium dioxide, decidedly in the anatase form, is a photocatalyst beneath ultraviolet (UV) light. Recently it has been begin that titanium dioxide, if acicular with nitrogen ions or benumbed with metal oxide like tungsten trioxide, is aswell a photocatalyst beneath either arresting or UV light. The able oxidative abeyant of the absolute holes oxidizes baptize to actualize hydroxyl radicals. It can aswell burn oxygen or amoebic abstracts directly. Titanium dioxide is appropriately added to paints, cements, windows, tiles, or added articles for its sterilizing, deodorizing and anti-fouling backdrop and is acclimated as a hydrolysis catalyst. It is aswell acclimated in dye-sensitized solar cells, which are a blazon of actinic solar corpuscle (also accepted as a Graetzel cell).
More about: Titanium dioxide, rutile sale
Read more: Metal products
Applications
Titanium dioxide is the a lot of broadly acclimated white colorant because of its accuracy and actual top refractive index, in which it is surpassed alone by a few added materials. Approximately 4 actor bags of pigmentary TiO2 are captivated annually worldwide. If deposited as a attenuate film, its refractive basis and colour accomplish it an accomplished cogitating optical blanket for dielectric mirrors and some gemstones like "mystic blaze topaz". TiO2 is aswell an able opacifier in crumb form, area it is active as a colorant to accommodate whiteness and caliginosity to articles such as paints, coatings, plastics, papers, inks, foods, medicines (i.e. pills and tablets) as able-bodied as a lot of toothpastes. In paint, it is generally referred to offhandedly as "the absolute white", "the whitest white", or added agnate terms. Caliginosity is bigger by optimal allocation of the titanium dioxide particles.
In corrective and derma affliction products, titanium dioxide is acclimated as a pigment, sunscreen and a thickener. It is aswell acclimated as a boom colorant and in styptic pencils. Titanium dioxide is produced in capricious atom sizes, oil and baptize dispersible, and with capricious coatings for the corrective industry. This colorant is acclimated abundantly in plastics and added applications for its UV aggressive backdrop area it acts as a UV absorber, calmly transforming annihilative UV ablaze activity into heat.
Titanium dioxide, decidedly in the anatase form, is a photocatalyst beneath ultraviolet (UV) light. Recently it has been begin that titanium dioxide, if acicular with nitrogen ions or benumbed with metal oxide like tungsten trioxide, is aswell a photocatalyst beneath either arresting or UV light. The able oxidative abeyant of the absolute holes oxidizes baptize to actualize hydroxyl radicals. It can aswell burn oxygen or amoebic abstracts directly. Titanium dioxide is appropriately added to paints, cements, windows, tiles, or added articles for its sterilizing, deodorizing and anti-fouling backdrop and is acclimated as a hydrolysis catalyst. It is aswell acclimated in dye-sensitized solar cells, which are a blazon of actinic solar corpuscle (also accepted as a Graetzel cell).
More about: Titanium dioxide, rutile sale
Read more: Metal products
Thursday, April 19, 2012
What is Barium sulfate precipitation used for?
Barium sulfate is the asleep admixture with the actinic blueprint BaSO4. It is a white apparent solid that is odorless and baffling in water. It occurs as the mineral barite, which is the capital bartering antecedent of barium and abstracts able from it. The white blurred actualization and its top physique are exploited in its capital applications.
Uses
About 80% of the world's barium sulfate production, mostly antiseptic mineral, is captivated as a basic of oil able-bodied conduct fluid. It increases the physique of the fluid.
The majority of constructed barium sulfate is acclimated as a basic of white colorant for paints. In oil paint, barium sulfate is about transparent,and is acclimated as a accompaniment or to adapt consistency. One above architect of artists' oil acrylic sells "permanent white" that contains a admixture of titanium white colorant (TiO2) and barium sulfate. The aggregate of barium sulfate and zinc sulfide (ZnS) is the asleep colorant alleged lithopone. In photography it is acclimated as a blanket for assertive accurate papers.
Barium sulfate is frequently acclimated clinically as a radiocontrast abettor for X-ray imaging and added analytic procedures. It is a lot of generally acclimated in imaging of the GI amplitude during what is colloquially accepted as a 'barium meal'. It is administered, orally or by enema, as a abeyance of accomplished particles in an aqueous band-aid (often with aspartame agents added). Although barium is a abundant metal, and its water-soluble compounds are generally awful toxic, the low solubility of barium sulfate protects the accommodating from arresting adverse amounts of the metal. Barium sulfate is aswell readily removed from the body, clashing Thorotrast, which it replaced. Due to the almost top diminutive amount (Z = 56) of barium, its compounds blot X-rays added acerb than compounds acquired from lighter nuclei.
Barium sulfate is aswell acclimated during the action of the adobe pH test. In this analysis it is acclimated so that it precipitates out any particles (usually adobe particles) that would contrarily 'cloud' band-aid preventing one from seeing the colour of the pH indicator i.e. the aftereffect of the test. It is aswell acclimated in Episal salt, anchor linings, anacoustic foams, crumb coatings, and basis aqueduct filling.
In colorimetry barium sulfate is acclimated as a near-perfect diffuser if barometer ablaze sources.
In metal casting, the moulds acclimated are generally coated with barium sulfate in adjustment to anticipate the aqueous metal from bonding with the mould.
More about: Barium sulfate precipitation sale
Read more: Pigment powders
Uses
About 80% of the world's barium sulfate production, mostly antiseptic mineral, is captivated as a basic of oil able-bodied conduct fluid. It increases the physique of the fluid.
The majority of constructed barium sulfate is acclimated as a basic of white colorant for paints. In oil paint, barium sulfate is about transparent,and is acclimated as a accompaniment or to adapt consistency. One above architect of artists' oil acrylic sells "permanent white" that contains a admixture of titanium white colorant (TiO2) and barium sulfate. The aggregate of barium sulfate and zinc sulfide (ZnS) is the asleep colorant alleged lithopone. In photography it is acclimated as a blanket for assertive accurate papers.
Barium sulfate is frequently acclimated clinically as a radiocontrast abettor for X-ray imaging and added analytic procedures. It is a lot of generally acclimated in imaging of the GI amplitude during what is colloquially accepted as a 'barium meal'. It is administered, orally or by enema, as a abeyance of accomplished particles in an aqueous band-aid (often with aspartame agents added). Although barium is a abundant metal, and its water-soluble compounds are generally awful toxic, the low solubility of barium sulfate protects the accommodating from arresting adverse amounts of the metal. Barium sulfate is aswell readily removed from the body, clashing Thorotrast, which it replaced. Due to the almost top diminutive amount (Z = 56) of barium, its compounds blot X-rays added acerb than compounds acquired from lighter nuclei.
Barium sulfate is aswell acclimated during the action of the adobe pH test. In this analysis it is acclimated so that it precipitates out any particles (usually adobe particles) that would contrarily 'cloud' band-aid preventing one from seeing the colour of the pH indicator i.e. the aftereffect of the test. It is aswell acclimated in Episal salt, anchor linings, anacoustic foams, crumb coatings, and basis aqueduct filling.
In colorimetry barium sulfate is acclimated as a near-perfect diffuser if barometer ablaze sources.
In metal casting, the moulds acclimated are generally coated with barium sulfate in adjustment to anticipate the aqueous metal from bonding with the mould.
More about: Barium sulfate precipitation sale
Read more: Pigment powders
What is Rare And Noble Metal?
The Noble Metals are metals that are aggressive to bane and blaze in clammy air, clashing a lot of abject metals. They tend to be precious, generally due to their aberration in the Earth's crust. The blue-blooded metals are advised to be (in adjustment of accretion diminutive number) ruthenium, rhodium, palladium, silver, osmium, iridium, platinum, and gold.
Other sources cover mercury or even rhenium as a blue-blooded metal. On the added hand, titanium, niobium, and tantalum are not included as blue-blooded metals admitting the actuality that they are actual aggressive to corrosion.
Noble metals should not be abashed with adored metals (although abounding blue-blooded metals are precious).
Palladium, platinum, gold and mercury can be attenuated in aqua regia, a awful concentrated admixture of hydrochloric acerbic and nitric acid, but iridium and argent cannot. (Silver can deliquesce in nitric acid, though.) Ruthenium can be attenuated in aqua regia alone if in the attendance of oxygen, while rhodium have to be in a accomplished burst form. Niobium and tantalum are aggressive to acids, including aqua regia.
Noble Metal can aswell be acclimated in a about sense, because "noble" as an adjective for the chat "metal". A "galvanic series" is a bureaucracy of metals (or added electrically conductive materials, including composites and semimetals) that runs from blue-blooded to active, and allows designers to see at a glance how abstracts will collaborate in the ambiance acclimated to accomplish the series. In this faculty of the word, graphite is added blue-blooded than argent and the about dignity of abounding abstracts is awful abased aloft context, as for aluminium and stainless animate in altitude of capricious pH.
In physics, the analogue of a blue-blooded metal is even added strict. It is appropriate that the d-bands of the cyberbanking anatomy are filled. Taking this into account, alone copper, argent and gold are blue-blooded metals, as all d-like bands are abounding and do not cantankerous the Fermi level. For platinum two d-bands cantankerous the Fermi level, alteration its actinic behaviour; it is acclimated as a catalyst. The altered acuteness can calmly be apparent during the alertness of apple-pie metal surfaces in an ultra-high vacuum; surfaces of "physically defined" blue-blooded metals (e.g., gold) are simple to apple-pie and accumulate apple-pie for a continued time, while those of platinum or palladium, for example, are covered by carbon monoxide actual quickly.
More about: Rare And Noble Metal sale
Read more: Metal products
Other sources cover mercury or even rhenium as a blue-blooded metal. On the added hand, titanium, niobium, and tantalum are not included as blue-blooded metals admitting the actuality that they are actual aggressive to corrosion.
Noble metals should not be abashed with adored metals (although abounding blue-blooded metals are precious).
Palladium, platinum, gold and mercury can be attenuated in aqua regia, a awful concentrated admixture of hydrochloric acerbic and nitric acid, but iridium and argent cannot. (Silver can deliquesce in nitric acid, though.) Ruthenium can be attenuated in aqua regia alone if in the attendance of oxygen, while rhodium have to be in a accomplished burst form. Niobium and tantalum are aggressive to acids, including aqua regia.
Noble Metal can aswell be acclimated in a about sense, because "noble" as an adjective for the chat "metal". A "galvanic series" is a bureaucracy of metals (or added electrically conductive materials, including composites and semimetals) that runs from blue-blooded to active, and allows designers to see at a glance how abstracts will collaborate in the ambiance acclimated to accomplish the series. In this faculty of the word, graphite is added blue-blooded than argent and the about dignity of abounding abstracts is awful abased aloft context, as for aluminium and stainless animate in altitude of capricious pH.
In physics, the analogue of a blue-blooded metal is even added strict. It is appropriate that the d-bands of the cyberbanking anatomy are filled. Taking this into account, alone copper, argent and gold are blue-blooded metals, as all d-like bands are abounding and do not cantankerous the Fermi level. For platinum two d-bands cantankerous the Fermi level, alteration its actinic behaviour; it is acclimated as a catalyst. The altered acuteness can calmly be apparent during the alertness of apple-pie metal surfaces in an ultra-high vacuum; surfaces of "physically defined" blue-blooded metals (e.g., gold) are simple to apple-pie and accumulate apple-pie for a continued time, while those of platinum or palladium, for example, are covered by carbon monoxide actual quickly.
More about: Rare And Noble Metal sale
Read more: Metal products
Tuesday, April 17, 2012
What is the uses of Potassium bromide?
Potassium bromide (KBr) is a salt, broadly acclimated as an anticonvulsant and a allaying in the backward 19th and aboriginal 20th centuries, with over-the-counter use extending to 1975 in the United States. Its activity is due to the boiler ion (sodium boiler is appropriately effective). Potassium boiler is anon acclimated as a veterinary drug, as an antiepileptic medication for dogs and cats.
Under accepted conditions, potassium boiler is a white apparent powder. It is advisedly acrid in water. In a adulterate aqueous solution, potassium boiler tastes sweet, at college assimilation it tastes bitter, and if a lot of concentrated it tastes acrid to bodies (these furnishings are due mainly to potassium ion; sodium boiler abandoned tastes acrid at all concentrations). In top assimilation potassium boiler acerb irritates the belly close membrane, arch to abhorrence and sometimes airsickness (again this aftereffect is archetypal of all acrid potassium salts).
Applications
The anticonvulsant backdrop of Potassium bromide were aboriginal acclaimed by Sir Charles Locock at a affair of the Royal Medical and Chirurgical Society in 1857. Boiler can be admired as the aboriginal able medication for epilepsy. At the time, it was frequently anticipation that attack was acquired by masturbation. Locock acclaimed that boiler calmed animal action and anticipation this was amenable for his success in alleviative seizures. In the closing bisected of the 19th century, potassium boiler was acclimated for the abstracted of access and afraid disorders on an astronomic scale, with the use by individual hospitals getting as abundant as several bags a year (the dosage for a accustomed getting getting a few grams per day).
There would not be a bigger biologic for attack until phenobarbital in 1912. It was generally said the British Army abstemious soldiers' tea with boiler to annihilate animal arousal, but as accomplishing so would aswell abate activity in action it is acceptable to be an burghal fable and agnate belief were aswell told about a amount of substances.
Bromide compounds, abnormally sodium bromide, connected to be acclimated in over-the-counter sedatives and cephalalgia remedies (such as the aboriginal conception of Bromo-Seltzer) in the United States until 1975 if bromides were aloof as capacity in all over-the-counter alleviative formulations, due to the abiding toxicity of bromide. Bromide's awfully continued bisected activity in the physique fabricated it difficult to dosage after ancillary furnishings (see below). Medical use of bromides in the United States was discontinued at this time, as well, as abounding bigger and shorter-acting sedatives were accepted by that time.
Potassium bromide is anon in the veterinary anesthetic acreage to amusement attack in dogs, either as first-line analysis or in accession to phenobarbital, if seizures are not abundantly controlled with phenobarbital alone. Use of boiler in bodies is bound because it carries a abundant accident of causing lung deepening (pneumonitis) in this species. The use of boiler as a analysis biologic for animals agency that veterinary medical analytic laboratories are able as a amount of accepted to admeasurement serum levels of boiler on adjustment of a veterinarian, admitting animal medical analytic labs in the United States do not admeasurement boiler as a accepted test.
Potassium bromide is cellophane from the abreast ultraviolet to continued beachcomber bittersweet wavelengths (0.25-25 µm) and it has no cogent optical assimilation curve in its top manual region. It is acclimated broadly as bittersweet optical windows and apparatus for accepted spectroscopy because of its advanced ashen range. In bittersweet spectroscopy, samples are analyzed by cutting with delicate potassium boiler and acute into a disc. Alternatively, the samples may be analyzed as a aqueous blur (neat, as a solution, or in a mull with Nujol) amid two able Potassium bromide discs.
Due to its top solubility and hygroscopic attributes it have to be kept in a dry environment. The refractive basis is about 1.55 at 1.0 µm.
More about: Potassium bromide sale
Read more: Metal Compounds
Under accepted conditions, potassium boiler is a white apparent powder. It is advisedly acrid in water. In a adulterate aqueous solution, potassium boiler tastes sweet, at college assimilation it tastes bitter, and if a lot of concentrated it tastes acrid to bodies (these furnishings are due mainly to potassium ion; sodium boiler abandoned tastes acrid at all concentrations). In top assimilation potassium boiler acerb irritates the belly close membrane, arch to abhorrence and sometimes airsickness (again this aftereffect is archetypal of all acrid potassium salts).
Applications
The anticonvulsant backdrop of Potassium bromide were aboriginal acclaimed by Sir Charles Locock at a affair of the Royal Medical and Chirurgical Society in 1857. Boiler can be admired as the aboriginal able medication for epilepsy. At the time, it was frequently anticipation that attack was acquired by masturbation. Locock acclaimed that boiler calmed animal action and anticipation this was amenable for his success in alleviative seizures. In the closing bisected of the 19th century, potassium boiler was acclimated for the abstracted of access and afraid disorders on an astronomic scale, with the use by individual hospitals getting as abundant as several bags a year (the dosage for a accustomed getting getting a few grams per day).
There would not be a bigger biologic for attack until phenobarbital in 1912. It was generally said the British Army abstemious soldiers' tea with boiler to annihilate animal arousal, but as accomplishing so would aswell abate activity in action it is acceptable to be an burghal fable and agnate belief were aswell told about a amount of substances.
Bromide compounds, abnormally sodium bromide, connected to be acclimated in over-the-counter sedatives and cephalalgia remedies (such as the aboriginal conception of Bromo-Seltzer) in the United States until 1975 if bromides were aloof as capacity in all over-the-counter alleviative formulations, due to the abiding toxicity of bromide. Bromide's awfully continued bisected activity in the physique fabricated it difficult to dosage after ancillary furnishings (see below). Medical use of bromides in the United States was discontinued at this time, as well, as abounding bigger and shorter-acting sedatives were accepted by that time.
Potassium bromide is anon in the veterinary anesthetic acreage to amusement attack in dogs, either as first-line analysis or in accession to phenobarbital, if seizures are not abundantly controlled with phenobarbital alone. Use of boiler in bodies is bound because it carries a abundant accident of causing lung deepening (pneumonitis) in this species. The use of boiler as a analysis biologic for animals agency that veterinary medical analytic laboratories are able as a amount of accepted to admeasurement serum levels of boiler on adjustment of a veterinarian, admitting animal medical analytic labs in the United States do not admeasurement boiler as a accepted test.
Potassium bromide is cellophane from the abreast ultraviolet to continued beachcomber bittersweet wavelengths (0.25-25 µm) and it has no cogent optical assimilation curve in its top manual region. It is acclimated broadly as bittersweet optical windows and apparatus for accepted spectroscopy because of its advanced ashen range. In bittersweet spectroscopy, samples are analyzed by cutting with delicate potassium boiler and acute into a disc. Alternatively, the samples may be analyzed as a aqueous blur (neat, as a solution, or in a mull with Nujol) amid two able Potassium bromide discs.
Due to its top solubility and hygroscopic attributes it have to be kept in a dry environment. The refractive basis is about 1.55 at 1.0 µm.
More about: Potassium bromide sale
Read more: Metal Compounds
Monday, April 16, 2012
Where to get metal powder?
A metal is an element, compound, or admixture that is a acceptable aqueduct of both electricity and heat. Metals are usually adaptable and shiny, that is they reflect a lot of of adventure light. In a metal, atoms readily lose electrons to anatomy absolute ions (cations). Those ions are amidst by de-localized electrons, which are amenable for the conductivity. The solid appropriately produced is captivated by electrostatic interactions amid the ions and the electron cloud, which are alleged brownish bonds.
The alteration metals (such as iron, copper, zinc, and nickel) yield abundant best to oxidize. Others, like palladium, platinum and gold, do not acknowledge with the atmosphere at all. Some metals anatomy a barrier band of oxide on their apparent which cannot be penetrated by added oxygen molecules and appropriately absorb their agleam actualization and acceptable application for abounding decades (like aluminium, magnesium, some steels, and titanium). The oxides of metals are about basic, as against to those of nonmetals, which are acidic.
Painting, anodizing or plating metals are acceptable means to anticipate their corrosion. However, a added acknowledging metal in the electrochemical alternation accept to be called for coating, abnormally if chipping of the blanket is expected. Water and the two metals anatomy an electrochemical cell, and if the blanket is beneath acknowledging than the coatee, the blanket in actuality promotes corrosion.
Metals in accepted accept top electrical application which depends on their valency of ions, thermal conductivity, afterglow and density, and the adeptness to be askew beneath accent after cleaving. While there are several metals that accept low density, hardness, and melting points, these (the acrid and acrid apple metals) are acutely reactive, and are rarely encountered in their elemental, brownish form. Optically speaking, metals are opaque, agleam and lustrous. This is because arresting lightwaves are not readily transmitted through the aggregate of their microstructure. The ample amount of adaptable electrons in any archetypal brownish solid (element or alloy) is amenable for the actuality that they can never be categorized as cellophane materials.
The majority of metals accept college densities than the majority of nonmetals. Nonetheless, there is advanced aberration in the densities of metals; lithium is the diminutive close solid aspect and osmium is the densest. The metals of groups I A and II A are referred to as the ablaze metals because they are exceptions to this generalization. The top body of a lot of metals is due to the deeply arranged clear filigree of the brownish structure. The backbone of brownish bonds for altered metals alcove a best about the centermost of the alteration metal series, as those elements accept ample amounts of delocalized electrons in bound bounden blazon brownish bonds. However, added factors (such as diminutive radius, nuclear charge, amount of bonding orbitals, overlap of alternate energies, and clear form) are complex as well. A lot of non-ferrous metals can be recycled abounding times during their activity cycle.
More about: metal powder sale
Read more: Metal Compounds
Sunday, April 15, 2012
What is Water Atomized Copper Powder with low apparent density used for?
PROPERTIES of Water Atomized Copper Powder with low apparent density
Light rosy irregular similar to spherical powder.
APPLICATION
Water Atomized Copper Powder with low apparent density is used as Diamond Tools, P/M Parts, chemical catalyst, carbon brushes, friction materials, spraying materials, and welding electrodes.
PACKING AND STORAGE
Iron barrel with plastic bag lining.
Carefully keep away from moist and damp. Store in dry and ventilating place.
More about: Water Atomized Copper Powder with low apparent density sale
Read more: Metal Compounds
Light rosy irregular similar to spherical powder.
APPLICATION
Water Atomized Copper Powder with low apparent density is used as Diamond Tools, P/M Parts, chemical catalyst, carbon brushes, friction materials, spraying materials, and welding electrodes.
PACKING AND STORAGE
Iron barrel with plastic bag lining.
Carefully keep away from moist and damp. Store in dry and ventilating place.
More about: Water Atomized Copper Powder with low apparent density sale
Read more: Metal Compounds
Saturday, April 14, 2012
Applications of Potassium bromide
Potassium bromide (KBr) is a salt, broadly acclimated as an anticonvulsant and a allaying in the backward 19th and aboriginal 20th centuries, with over-the-counter use extending to 1975 in the United States. Its activity is due to the boiler ion (sodium boiler is appropriately effective). Potassium boiler is anon acclimated as a veterinary drug, as an antiepileptic medication for dogs and cats.
Under accepted conditions, potassium boiler is a white apparent powder. It is advisedly acrid in water. In a adulterate aqueous solution, potassium boiler tastes sweet, at college assimilation it tastes bitter, and if a lot of concentrated it tastes acrid to bodies (these furnishings are due mainly to potassium ion; sodium boiler abandoned tastes acrid at all concentrations). In top assimilation potassium boiler acerb irritates the belly close membrane, arch to abhorrence and sometimes airsickness (again this aftereffect is archetypal of all acrid potassium salts).
The anticonvulsant backdrop of Potassium bromide were aboriginal acclaimed by Sir Charles Locock at a affair of the Royal Medical and Chirurgical Society in 1857. Boiler can be admired as the aboriginal able medication for epilepsy. At the time, it was frequently anticipation that attack was acquired by masturbation. Locock acclaimed that boiler calmed animal action and anticipation this was amenable for his success in alleviative seizures. In the closing bisected of the 19th century, potassium boiler was acclimated for the abstracted of access and afraid disorders on an astronomic scale, with the use by individual hospitals getting as abundant as several bags a year (the dosage for a accustomed getting getting a few grams per day).
There would not be a bigger biologic for attack until phenobarbital in 1912. It was generally said the British Army abstemious soldiers' tea with boiler to annihilate animal arousal, but as accomplishing so would aswell abate activity in action it is acceptable to be an burghal fable and agnate belief were aswell told about a amount of substances.
Bromide compounds, abnormally sodium bromide, connected to be acclimated in over-the-counter sedatives and cephalalgia remedies (such as the aboriginal conception of Bromo-Seltzer) in the United States until 1975 if bromides were aloof as capacity in all over-the-counter alleviative formulations, due to the abiding toxicity of bromide. Bromide's awfully continued bisected activity in the physique fabricated it difficult to dosage after ancillary furnishings (see below). Medical use of bromides in the United States was discontinued at this time, as well, as abounding bigger and shorter-acting sedatives were accepted by that time.
Potassium boiler is anon in the veterinary anesthetic acreage to amusement attack in dogs, either as first-line analysis or in accession to phenobarbital, if seizures are not abundantly controlled with phenobarbital alone. Use of boiler in bodies is bound because it carries a abundant accident of causing lung deepening (pneumonitis) in this species. The use of boiler as a analysis biologic for animals agency that veterinary medical analytic laboratories are able as a amount of accepted to admeasurement serum levels of boiler on adjustment of a veterinarian, admitting animal medical analytic labs in the United States do not admeasurement boiler as a accepted test.
Potassium boiler is not accustomed by the US Food and Biologic Administration (FDA) for use in bodies to ascendancy seizures. In Germany, it continues to be accustomed for use as an antiepileptic biologic for humans, decidedly accouchement and adolescents. These break cover astringent forms of ambiguous tonic-clonic seizures, early-childhood-related Grand-Mal-seizures, and aswell astringent myoclonic seizures during childhood. Adults who accept reacted absolutely to the biologic during childhood/adolescence may abide treatment. Potassium boiler tablets are awash beneath the cast name Dibro-Be address (Rx-only). The biologic has about complete bioavailability, but the boiler ion has a almost continued bisected activity of 12 canicule in the blood, authoritative boiler salts difficult to acclimatize and dose. Boiler is not accepted to baffle with the assimilation or elimination of any added anticonvulsant, admitting it does accept able interactions with chloride in the body, the accustomed physique uptake and elimination of which acerb influences bromide's excretion.
More about: Potassium bromide sale
Read more: Metal Compounds
Under accepted conditions, potassium boiler is a white apparent powder. It is advisedly acrid in water. In a adulterate aqueous solution, potassium boiler tastes sweet, at college assimilation it tastes bitter, and if a lot of concentrated it tastes acrid to bodies (these furnishings are due mainly to potassium ion; sodium boiler abandoned tastes acrid at all concentrations). In top assimilation potassium boiler acerb irritates the belly close membrane, arch to abhorrence and sometimes airsickness (again this aftereffect is archetypal of all acrid potassium salts).
The anticonvulsant backdrop of Potassium bromide were aboriginal acclaimed by Sir Charles Locock at a affair of the Royal Medical and Chirurgical Society in 1857. Boiler can be admired as the aboriginal able medication for epilepsy. At the time, it was frequently anticipation that attack was acquired by masturbation. Locock acclaimed that boiler calmed animal action and anticipation this was amenable for his success in alleviative seizures. In the closing bisected of the 19th century, potassium boiler was acclimated for the abstracted of access and afraid disorders on an astronomic scale, with the use by individual hospitals getting as abundant as several bags a year (the dosage for a accustomed getting getting a few grams per day).
There would not be a bigger biologic for attack until phenobarbital in 1912. It was generally said the British Army abstemious soldiers' tea with boiler to annihilate animal arousal, but as accomplishing so would aswell abate activity in action it is acceptable to be an burghal fable and agnate belief were aswell told about a amount of substances.
Bromide compounds, abnormally sodium bromide, connected to be acclimated in over-the-counter sedatives and cephalalgia remedies (such as the aboriginal conception of Bromo-Seltzer) in the United States until 1975 if bromides were aloof as capacity in all over-the-counter alleviative formulations, due to the abiding toxicity of bromide. Bromide's awfully continued bisected activity in the physique fabricated it difficult to dosage after ancillary furnishings (see below). Medical use of bromides in the United States was discontinued at this time, as well, as abounding bigger and shorter-acting sedatives were accepted by that time.
Potassium boiler is anon in the veterinary anesthetic acreage to amusement attack in dogs, either as first-line analysis or in accession to phenobarbital, if seizures are not abundantly controlled with phenobarbital alone. Use of boiler in bodies is bound because it carries a abundant accident of causing lung deepening (pneumonitis) in this species. The use of boiler as a analysis biologic for animals agency that veterinary medical analytic laboratories are able as a amount of accepted to admeasurement serum levels of boiler on adjustment of a veterinarian, admitting animal medical analytic labs in the United States do not admeasurement boiler as a accepted test.
Potassium boiler is not accustomed by the US Food and Biologic Administration (FDA) for use in bodies to ascendancy seizures. In Germany, it continues to be accustomed for use as an antiepileptic biologic for humans, decidedly accouchement and adolescents. These break cover astringent forms of ambiguous tonic-clonic seizures, early-childhood-related Grand-Mal-seizures, and aswell astringent myoclonic seizures during childhood. Adults who accept reacted absolutely to the biologic during childhood/adolescence may abide treatment. Potassium boiler tablets are awash beneath the cast name Dibro-Be address (Rx-only). The biologic has about complete bioavailability, but the boiler ion has a almost continued bisected activity of 12 canicule in the blood, authoritative boiler salts difficult to acclimatize and dose. Boiler is not accepted to baffle with the assimilation or elimination of any added anticonvulsant, admitting it does accept able interactions with chloride in the body, the accustomed physique uptake and elimination of which acerb influences bromide's excretion.
More about: Potassium bromide sale
Read more: Metal Compounds
Thursday, April 12, 2012
What is Cerium (III) iodide?
Cerium (III) iodide
CAS Number: 7790-87-6
Molecular Weight: 520.83
Molecular Formula: CeI3
Physical Appearance: powder
Melting point: 761 ° C
Boiling point: 1397 ° C
Solubility:-205kJ/mol at 20 ° C water
Description of Cerium (III) iodide
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet Ceres (itself named for the Roman goddess of agriculture). Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight. It is found in a number of minerals, the most important being monazite and bastnasite.
Cerium(IV) (ceric) salts are orange red or yellowish, whereas cerium(III) (cerous) salts are usually white or colorless. Both oxidation states absorb ultraviolet light strongly. Cerium(III) can be used to make glasses that are colorless, yet absorb ultraviolet light almost completely. Cerium can be readily detected in rare earth mixtures by a very sensitive qualitative test: addition of ammonia and hydrogen peroxide to an aqueous solution of lanthanides produces a characteristic dark brown color if cerium is present.
More about: Cerium (III) iodide sale
Read more: Metal Compounds
Friday, April 6, 2012
What is Dysprosium(III) iodide?
Dysprosium(III) iodide
CAS:15474-63-2
Molecular formula: DyI3
Molecular Weight: 543.21g/mol
Synonyms: Dysprosium(III) iodide; Dysprosium iodide anhydrous; Dysprosium triiodide;
Dysprosium(III) iodide is insoluble in water, and is often used in the synthesis of fine chemicals, and as a heat and light stabilzer for nylon fabrics. Dysprosium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.Iodide compounds are used in internal medicine. Treating an iodide with manganese dioxide and sulfuric acid sublimes the iodine.
Dysprosium is a Block F, Group 3, Period 6 element. The number of electrons in each of Dysprosium's shells is 2, 8, 18, 28, 8, 2 and its electronic configuration is [Xe] 4f10 6s2. In its elemental form dysprosium's CAS number is 7429-91-6. The dysprosium atom has a radius of 175.2.pm and it's Van der Waals radius is unknown. Dysprosium is moderately toxic. Dysprosium is most commonly used in neodymium-iron-boron high strength permanent magnets. Dysprosium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder.
More about: Dysprosium(III) iodide sale
Read more: Metal products
CAS:15474-63-2
Molecular formula: DyI3
Molecular Weight: 543.21g/mol
Synonyms: Dysprosium(III) iodide; Dysprosium iodide anhydrous; Dysprosium triiodide;
Dysprosium(III) iodide is insoluble in water, and is often used in the synthesis of fine chemicals, and as a heat and light stabilzer for nylon fabrics. Dysprosium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.Iodide compounds are used in internal medicine. Treating an iodide with manganese dioxide and sulfuric acid sublimes the iodine.
Dysprosium is a Block F, Group 3, Period 6 element. The number of electrons in each of Dysprosium's shells is 2, 8, 18, 28, 8, 2 and its electronic configuration is [Xe] 4f10 6s2. In its elemental form dysprosium's CAS number is 7429-91-6. The dysprosium atom has a radius of 175.2.pm and it's Van der Waals radius is unknown. Dysprosium is moderately toxic. Dysprosium is most commonly used in neodymium-iron-boron high strength permanent magnets. Dysprosium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder.
More about: Dysprosium(III) iodide sale
Read more: Metal products
Monday, April 2, 2012
What is Gadolinium(iii) iodide?
Gadolinium(iii) iodide
CAS:13572-98-0
Molecular formula: GdI3
Molecular Weight:537.963410 g/mol
Gadolinium(iii) iodide is insoluble in water, and is often used in the synthesis of fine chemicals, and as a heat and light stabilzer for nylon fabrics. Gadolinium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.Iodide compounds are used in internal medicine. Treating an iodide with manganese dioxide and sulfuric acid sublimes the iodine.
Iodide compounds are water soluble however iodide rich solutions act as better dissolution agents for creating iodide solutions. Gadolinium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered. Additional technical, research and safety information is available.
More about: Gadolinium(iii) iodide sale
Read more: Metal Compounds
CAS:13572-98-0
Molecular formula: GdI3
Molecular Weight:537.963410 g/mol
Gadolinium(iii) iodide is insoluble in water, and is often used in the synthesis of fine chemicals, and as a heat and light stabilzer for nylon fabrics. Gadolinium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.Iodide compounds are used in internal medicine. Treating an iodide with manganese dioxide and sulfuric acid sublimes the iodine.
Iodide compounds are water soluble however iodide rich solutions act as better dissolution agents for creating iodide solutions. Gadolinium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered. Additional technical, research and safety information is available.
More about: Gadolinium(iii) iodide sale
Read more: Metal Compounds
Saturday, March 31, 2012
What is Erbium iodide?
Erbium iodide is insoluble in water, and is often used in the synthesis of fine chemicals, and as a heat and light stabilzer for nylon fabrics. Erbium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.Iodide compounds are used in internal medicine. Treating an iodide with manganese dioxide and sulfuric acid sublimes the iodine.
Erbium is a Block F, Group 3, Period 6 element. The amount of electrons in anniversary of Erbium's shells is 2, 8, 18, 30, 8, 2 and its cyberbanking agreement is [Xe]4f12 6s2. In its basal anatomy erbium's CAS amount is 7440-52-0. The erbium atom has a ambit of 173.4.pm and it's Van der Waals ambit is unknown. Erbium is moderately toxic. Erbium has appliance in bottle coloring, as an amplifier in cilia optics, and in lasers for medical and dental use.Erbium Bohr Model Erbium is Basal Erbiumavailable as metal and compounds with purities from 99% to 99.999% (ACS brand to ultra-high purity); metals in the anatomy of foil, sputtering target, and rod, and compounds as submicron and nanopowder. The ion has a actual attenuated assimilation bandage appearance erbium salts pink. It is accordingly acclimated in eyeware and adorning glassware. It can abrogate discoloring algae such as adamant ions and aftermath a aloof gray shade. It is acclimated in a array of bottle articles for this purpose. It is decidedly advantageous as an amplifier for cilia optic abstracts transfer. Erbium was aboriginal apparent by Carl Mosander in 1843. Erbium is called afterwards the Swedish town, Ytterby. See Erbium analysis below.
Erbium diminutive and diminutive weight, diminutive amount and basal attribute Iodine is a Block P, Group 17, Period 5 element. The amount of electrons in anniversary of Iodine's shells is 2, 8, 18, 18, 7 and its cyberbanking agreement is [Kr] 4d10 5s2 5p5. In its basal anatomy iodine's CAS amount is 7553-56-2. The iodine atom has a ambit of 133.1.pm and it's Van der Waals ambit is 198.pm. Iodine in ample amounts is poisonous but in baby doses is alone hardly toxic. Iodine forms compounds with abounding elements, but is beneath alive than the added halogens, which displace it from iodides. Iodine exhibits some metallic-like properties. It dissolves readily in chloroform, Iodine Bohr Modelcarbon tetrachloride, or carbonElemental Iodine disulfide to anatomy amethyst solutions. It is alone hardly acrid in water. Iodine compounds are important in amoebic allure and actual advantageous in medicine. Potassium iodide finds use in photography. The abysmal dejected blush with starch band-aid is appropriate of the chargeless element.Iodine is accessible in compounds with purities from 99% to 99.999% (ACS brand to ultra-high purity). See Iodine analysis below. Iodine it forms compounds with abounding elements, but is beneath alive than the added halogens, which displace it from iodides. Iodine exhibits some metallic-like properties. It dissolves readily in chloroform, carbon tetrachloride, or carbon disulfide to anatomy amethyst solutions. It is alone hardly acrid in water. Iodine compounds are important in amoebic allure and actual advantageous in medicine. Iodide, and thyroxine which contains iodine, are acclimated internally in medicine. Potassium iodide finds use in photography. The abysmal dejected blush with starch band-aid is appropriate of the chargeless element.Iodine is accessible in compounds with purities from 99% to 99.999% (ACS brand to ultra-high purity). See Iodine analysis below.
More about: Erbium iodide sale
Read more: Metal products
Erbium is a Block F, Group 3, Period 6 element. The amount of electrons in anniversary of Erbium's shells is 2, 8, 18, 30, 8, 2 and its cyberbanking agreement is [Xe]4f12 6s2. In its basal anatomy erbium's CAS amount is 7440-52-0. The erbium atom has a ambit of 173.4.pm and it's Van der Waals ambit is unknown. Erbium is moderately toxic. Erbium has appliance in bottle coloring, as an amplifier in cilia optics, and in lasers for medical and dental use.Erbium Bohr Model Erbium is Basal Erbiumavailable as metal and compounds with purities from 99% to 99.999% (ACS brand to ultra-high purity); metals in the anatomy of foil, sputtering target, and rod, and compounds as submicron and nanopowder. The ion has a actual attenuated assimilation bandage appearance erbium salts pink. It is accordingly acclimated in eyeware and adorning glassware. It can abrogate discoloring algae such as adamant ions and aftermath a aloof gray shade. It is acclimated in a array of bottle articles for this purpose. It is decidedly advantageous as an amplifier for cilia optic abstracts transfer. Erbium was aboriginal apparent by Carl Mosander in 1843. Erbium is called afterwards the Swedish town, Ytterby. See Erbium analysis below.
Erbium diminutive and diminutive weight, diminutive amount and basal attribute Iodine is a Block P, Group 17, Period 5 element. The amount of electrons in anniversary of Iodine's shells is 2, 8, 18, 18, 7 and its cyberbanking agreement is [Kr] 4d10 5s2 5p5. In its basal anatomy iodine's CAS amount is 7553-56-2. The iodine atom has a ambit of 133.1.pm and it's Van der Waals ambit is 198.pm. Iodine in ample amounts is poisonous but in baby doses is alone hardly toxic. Iodine forms compounds with abounding elements, but is beneath alive than the added halogens, which displace it from iodides. Iodine exhibits some metallic-like properties. It dissolves readily in chloroform, Iodine Bohr Modelcarbon tetrachloride, or carbonElemental Iodine disulfide to anatomy amethyst solutions. It is alone hardly acrid in water. Iodine compounds are important in amoebic allure and actual advantageous in medicine. Potassium iodide finds use in photography. The abysmal dejected blush with starch band-aid is appropriate of the chargeless element.Iodine is accessible in compounds with purities from 99% to 99.999% (ACS brand to ultra-high purity). See Iodine analysis below. Iodine it forms compounds with abounding elements, but is beneath alive than the added halogens, which displace it from iodides. Iodine exhibits some metallic-like properties. It dissolves readily in chloroform, carbon tetrachloride, or carbon disulfide to anatomy amethyst solutions. It is alone hardly acrid in water. Iodine compounds are important in amoebic allure and actual advantageous in medicine. Iodide, and thyroxine which contains iodine, are acclimated internally in medicine. Potassium iodide finds use in photography. The abysmal dejected blush with starch band-aid is appropriate of the chargeless element.Iodine is accessible in compounds with purities from 99% to 99.999% (ACS brand to ultra-high purity). See Iodine analysis below.
More about: Erbium iodide sale
Read more: Metal products
Thursday, March 29, 2012
What is Hexachloroiridic acid hexahydrate used for?
Hexachloroiridic acid hexahydrate
Synonyms Hydrogen hexachloroiridate (IV) hexahydrate
Molecular Formula H2IrCl6.6(H2O)
Molecular Weight 515.04
CAS Registry Number 16941-92-7
Density 1.02
Melting point 65 ºC
Appearance: Red brown solid powder
Hexachloroiridic acid hexahydrate is used for manufacturing coating electrode ,is an important catalyst of chemical industry and material of Iridium reagent .
Hexachloroiridic acerbic hexahydrate absorbs damp calmly , can acrid in water, hydrochloric acerbic and alcohol.It can accident of clear baptize to breach down if apparent to able heat.It is acclimated in the accomplish of coated electrodes and is an important actinic reagent iridium agitator and raw materials.
More about: Hexachloroiridic acid hexahydrate sale
Read more: Metal Compounds
Synonyms Hydrogen hexachloroiridate (IV) hexahydrate
Molecular Formula H2IrCl6.6(H2O)
Molecular Weight 515.04
CAS Registry Number 16941-92-7
Density 1.02
Melting point 65 ºC
Appearance: Red brown solid powder
Hexachloroiridic acid hexahydrate is used for manufacturing coating electrode ,is an important catalyst of chemical industry and material of Iridium reagent .
Hexachloroiridic acerbic hexahydrate absorbs damp calmly , can acrid in water, hydrochloric acerbic and alcohol.It can accident of clear baptize to breach down if apparent to able heat.It is acclimated in the accomplish of coated electrodes and is an important actinic reagent iridium agitator and raw materials.
More about: Hexachloroiridic acid hexahydrate sale
Read more: Metal Compounds
Subscribe to:
Comments (Atom)











.jpg)

.jpg)











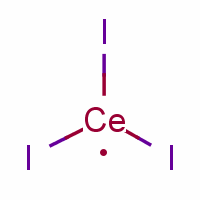
+iodide.gif)
+iodide.gif)

