Iron oxide is a actinic admixture that is fabricated of oxygen and iron. Adamant is a brownish aspect that is begin in about 5% of the Earth's crust. When adamant oxidizes, or rusts, colors such as yellow, orange, and red are created. A ample bulk of adamant oxide appears on the planet Mars, which is accepted as the "Red Planet." Mars appears to be red because its band is composed mostly of adamant oxide.
This admixture is frequently accepted as hematite. It is anticipation that adamant oxide deposits were created by the precipitation of adamant from sea baptize during the Proterozoic Eon, some 1.6 billion years ago. These deposits are begin in locations about the world, with the greatest concentrations getting in the USA, India, Australia, China, Brazil, and Russia.
There are three elements that are by itself magnetic: cobalt, nickel, and iron. Among those three, adamant is the a lot of magnetic, which is why adamant oxide is acclimated abundantly in the assembly of magnets, cyberbanking parts, audio and video cassette tapes, and ATM cards.
In the art world, Iron oxide is acclimated to actualize pigments such as burnt sienna and burnt umber. This adjustment of creating pigments in acrylic colors has been acclimated back the aged age. The cavern paintings at Lascaux are an archetype of how continued this admixture has been acclimated in the conception of art.
The cosmetics industry uses adamant oxide to actualize assorted pigments in make-up. Because it is non-toxic, baptize repellent, and it does not run or bleed, it is an ideal accretion to cosmetics such as mascara, foundation, and eye shadow. There are two altered types of compounds acclimated in the corrective industry: adamant oxide II which has a atramentous pigment, and adamant oxide III, which is red.
The altered pigments of this admixture are acclimated to dye such things as paint, concrete, leather, shoe polish, tiles, and rubber. Amber pigments can ambit in blush from a ablaze amber to a coffee blush brown. Red pigmentations ambit in blush from a abysmal orange to a abysmal red.
Iron oxide is aswell added to altered nutrients, feeds, and medications. It can be begin in assertive types of bloom products, such as talcum powder, facial cream, and physique cream. Some sunblock articles accommodate adamant oxide, which helps block UV application from damaging animal skin.
More about: Iron oxide blue sale
Read more: Pigment powders
Nonferrous metal, Metallic powders, Metal Compounds,Metal products from www.metal-powder-dust.com
Wednesday, April 25, 2012
Monday, April 23, 2012
Applications of Titanium dioxide, rutile
Titanium dioxide, aswell accepted as titanium(IV) oxide or titania, is the by itself occurring oxide of titanium, actinic blueprint TiO2. If acclimated as a pigment, it is alleged titanium white, Colorant White 6, or CI 77891. Generally it comes in two altered forms, rutile and anatase. It has a advanced ambit of applications, from acrylic to sunscreen to aliment colouring. If acclimated as a aliment colouring, it has E amount E171.
Applications
Titanium dioxide is the a lot of broadly acclimated white colorant because of its accuracy and actual top refractive index, in which it is surpassed alone by a few added materials. Approximately 4 actor bags of pigmentary TiO2 are captivated annually worldwide. If deposited as a attenuate film, its refractive basis and colour accomplish it an accomplished cogitating optical blanket for dielectric mirrors and some gemstones like "mystic blaze topaz". TiO2 is aswell an able opacifier in crumb form, area it is active as a colorant to accommodate whiteness and caliginosity to articles such as paints, coatings, plastics, papers, inks, foods, medicines (i.e. pills and tablets) as able-bodied as a lot of toothpastes. In paint, it is generally referred to offhandedly as "the absolute white", "the whitest white", or added agnate terms. Caliginosity is bigger by optimal allocation of the titanium dioxide particles.
In corrective and derma affliction products, titanium dioxide is acclimated as a pigment, sunscreen and a thickener. It is aswell acclimated as a boom colorant and in styptic pencils. Titanium dioxide is produced in capricious atom sizes, oil and baptize dispersible, and with capricious coatings for the corrective industry. This colorant is acclimated abundantly in plastics and added applications for its UV aggressive backdrop area it acts as a UV absorber, calmly transforming annihilative UV ablaze activity into heat.
Titanium dioxide, decidedly in the anatase form, is a photocatalyst beneath ultraviolet (UV) light. Recently it has been begin that titanium dioxide, if acicular with nitrogen ions or benumbed with metal oxide like tungsten trioxide, is aswell a photocatalyst beneath either arresting or UV light. The able oxidative abeyant of the absolute holes oxidizes baptize to actualize hydroxyl radicals. It can aswell burn oxygen or amoebic abstracts directly. Titanium dioxide is appropriately added to paints, cements, windows, tiles, or added articles for its sterilizing, deodorizing and anti-fouling backdrop and is acclimated as a hydrolysis catalyst. It is aswell acclimated in dye-sensitized solar cells, which are a blazon of actinic solar corpuscle (also accepted as a Graetzel cell).
More about: Titanium dioxide, rutile sale
Read more: Metal products
Applications
Titanium dioxide is the a lot of broadly acclimated white colorant because of its accuracy and actual top refractive index, in which it is surpassed alone by a few added materials. Approximately 4 actor bags of pigmentary TiO2 are captivated annually worldwide. If deposited as a attenuate film, its refractive basis and colour accomplish it an accomplished cogitating optical blanket for dielectric mirrors and some gemstones like "mystic blaze topaz". TiO2 is aswell an able opacifier in crumb form, area it is active as a colorant to accommodate whiteness and caliginosity to articles such as paints, coatings, plastics, papers, inks, foods, medicines (i.e. pills and tablets) as able-bodied as a lot of toothpastes. In paint, it is generally referred to offhandedly as "the absolute white", "the whitest white", or added agnate terms. Caliginosity is bigger by optimal allocation of the titanium dioxide particles.
In corrective and derma affliction products, titanium dioxide is acclimated as a pigment, sunscreen and a thickener. It is aswell acclimated as a boom colorant and in styptic pencils. Titanium dioxide is produced in capricious atom sizes, oil and baptize dispersible, and with capricious coatings for the corrective industry. This colorant is acclimated abundantly in plastics and added applications for its UV aggressive backdrop area it acts as a UV absorber, calmly transforming annihilative UV ablaze activity into heat.
Titanium dioxide, decidedly in the anatase form, is a photocatalyst beneath ultraviolet (UV) light. Recently it has been begin that titanium dioxide, if acicular with nitrogen ions or benumbed with metal oxide like tungsten trioxide, is aswell a photocatalyst beneath either arresting or UV light. The able oxidative abeyant of the absolute holes oxidizes baptize to actualize hydroxyl radicals. It can aswell burn oxygen or amoebic abstracts directly. Titanium dioxide is appropriately added to paints, cements, windows, tiles, or added articles for its sterilizing, deodorizing and anti-fouling backdrop and is acclimated as a hydrolysis catalyst. It is aswell acclimated in dye-sensitized solar cells, which are a blazon of actinic solar corpuscle (also accepted as a Graetzel cell).
More about: Titanium dioxide, rutile sale
Read more: Metal products
Thursday, April 19, 2012
What is Barium sulfate precipitation used for?
Barium sulfate is the asleep admixture with the actinic blueprint BaSO4. It is a white apparent solid that is odorless and baffling in water. It occurs as the mineral barite, which is the capital bartering antecedent of barium and abstracts able from it. The white blurred actualization and its top physique are exploited in its capital applications.
Uses
About 80% of the world's barium sulfate production, mostly antiseptic mineral, is captivated as a basic of oil able-bodied conduct fluid. It increases the physique of the fluid.
The majority of constructed barium sulfate is acclimated as a basic of white colorant for paints. In oil paint, barium sulfate is about transparent,and is acclimated as a accompaniment or to adapt consistency. One above architect of artists' oil acrylic sells "permanent white" that contains a admixture of titanium white colorant (TiO2) and barium sulfate. The aggregate of barium sulfate and zinc sulfide (ZnS) is the asleep colorant alleged lithopone. In photography it is acclimated as a blanket for assertive accurate papers.
Barium sulfate is frequently acclimated clinically as a radiocontrast abettor for X-ray imaging and added analytic procedures. It is a lot of generally acclimated in imaging of the GI amplitude during what is colloquially accepted as a 'barium meal'. It is administered, orally or by enema, as a abeyance of accomplished particles in an aqueous band-aid (often with aspartame agents added). Although barium is a abundant metal, and its water-soluble compounds are generally awful toxic, the low solubility of barium sulfate protects the accommodating from arresting adverse amounts of the metal. Barium sulfate is aswell readily removed from the body, clashing Thorotrast, which it replaced. Due to the almost top diminutive amount (Z = 56) of barium, its compounds blot X-rays added acerb than compounds acquired from lighter nuclei.
Barium sulfate is aswell acclimated during the action of the adobe pH test. In this analysis it is acclimated so that it precipitates out any particles (usually adobe particles) that would contrarily 'cloud' band-aid preventing one from seeing the colour of the pH indicator i.e. the aftereffect of the test. It is aswell acclimated in Episal salt, anchor linings, anacoustic foams, crumb coatings, and basis aqueduct filling.
In colorimetry barium sulfate is acclimated as a near-perfect diffuser if barometer ablaze sources.
In metal casting, the moulds acclimated are generally coated with barium sulfate in adjustment to anticipate the aqueous metal from bonding with the mould.
More about: Barium sulfate precipitation sale
Read more: Pigment powders
Uses
About 80% of the world's barium sulfate production, mostly antiseptic mineral, is captivated as a basic of oil able-bodied conduct fluid. It increases the physique of the fluid.
The majority of constructed barium sulfate is acclimated as a basic of white colorant for paints. In oil paint, barium sulfate is about transparent,and is acclimated as a accompaniment or to adapt consistency. One above architect of artists' oil acrylic sells "permanent white" that contains a admixture of titanium white colorant (TiO2) and barium sulfate. The aggregate of barium sulfate and zinc sulfide (ZnS) is the asleep colorant alleged lithopone. In photography it is acclimated as a blanket for assertive accurate papers.
Barium sulfate is frequently acclimated clinically as a radiocontrast abettor for X-ray imaging and added analytic procedures. It is a lot of generally acclimated in imaging of the GI amplitude during what is colloquially accepted as a 'barium meal'. It is administered, orally or by enema, as a abeyance of accomplished particles in an aqueous band-aid (often with aspartame agents added). Although barium is a abundant metal, and its water-soluble compounds are generally awful toxic, the low solubility of barium sulfate protects the accommodating from arresting adverse amounts of the metal. Barium sulfate is aswell readily removed from the body, clashing Thorotrast, which it replaced. Due to the almost top diminutive amount (Z = 56) of barium, its compounds blot X-rays added acerb than compounds acquired from lighter nuclei.
Barium sulfate is aswell acclimated during the action of the adobe pH test. In this analysis it is acclimated so that it precipitates out any particles (usually adobe particles) that would contrarily 'cloud' band-aid preventing one from seeing the colour of the pH indicator i.e. the aftereffect of the test. It is aswell acclimated in Episal salt, anchor linings, anacoustic foams, crumb coatings, and basis aqueduct filling.
In colorimetry barium sulfate is acclimated as a near-perfect diffuser if barometer ablaze sources.
In metal casting, the moulds acclimated are generally coated with barium sulfate in adjustment to anticipate the aqueous metal from bonding with the mould.
More about: Barium sulfate precipitation sale
Read more: Pigment powders
What is Rare And Noble Metal?
The Noble Metals are metals that are aggressive to bane and blaze in clammy air, clashing a lot of abject metals. They tend to be precious, generally due to their aberration in the Earth's crust. The blue-blooded metals are advised to be (in adjustment of accretion diminutive number) ruthenium, rhodium, palladium, silver, osmium, iridium, platinum, and gold.
Other sources cover mercury or even rhenium as a blue-blooded metal. On the added hand, titanium, niobium, and tantalum are not included as blue-blooded metals admitting the actuality that they are actual aggressive to corrosion.
Noble metals should not be abashed with adored metals (although abounding blue-blooded metals are precious).
Palladium, platinum, gold and mercury can be attenuated in aqua regia, a awful concentrated admixture of hydrochloric acerbic and nitric acid, but iridium and argent cannot. (Silver can deliquesce in nitric acid, though.) Ruthenium can be attenuated in aqua regia alone if in the attendance of oxygen, while rhodium have to be in a accomplished burst form. Niobium and tantalum are aggressive to acids, including aqua regia.
Noble Metal can aswell be acclimated in a about sense, because "noble" as an adjective for the chat "metal". A "galvanic series" is a bureaucracy of metals (or added electrically conductive materials, including composites and semimetals) that runs from blue-blooded to active, and allows designers to see at a glance how abstracts will collaborate in the ambiance acclimated to accomplish the series. In this faculty of the word, graphite is added blue-blooded than argent and the about dignity of abounding abstracts is awful abased aloft context, as for aluminium and stainless animate in altitude of capricious pH.
In physics, the analogue of a blue-blooded metal is even added strict. It is appropriate that the d-bands of the cyberbanking anatomy are filled. Taking this into account, alone copper, argent and gold are blue-blooded metals, as all d-like bands are abounding and do not cantankerous the Fermi level. For platinum two d-bands cantankerous the Fermi level, alteration its actinic behaviour; it is acclimated as a catalyst. The altered acuteness can calmly be apparent during the alertness of apple-pie metal surfaces in an ultra-high vacuum; surfaces of "physically defined" blue-blooded metals (e.g., gold) are simple to apple-pie and accumulate apple-pie for a continued time, while those of platinum or palladium, for example, are covered by carbon monoxide actual quickly.
More about: Rare And Noble Metal sale
Read more: Metal products
Other sources cover mercury or even rhenium as a blue-blooded metal. On the added hand, titanium, niobium, and tantalum are not included as blue-blooded metals admitting the actuality that they are actual aggressive to corrosion.
Noble metals should not be abashed with adored metals (although abounding blue-blooded metals are precious).
Palladium, platinum, gold and mercury can be attenuated in aqua regia, a awful concentrated admixture of hydrochloric acerbic and nitric acid, but iridium and argent cannot. (Silver can deliquesce in nitric acid, though.) Ruthenium can be attenuated in aqua regia alone if in the attendance of oxygen, while rhodium have to be in a accomplished burst form. Niobium and tantalum are aggressive to acids, including aqua regia.
Noble Metal can aswell be acclimated in a about sense, because "noble" as an adjective for the chat "metal". A "galvanic series" is a bureaucracy of metals (or added electrically conductive materials, including composites and semimetals) that runs from blue-blooded to active, and allows designers to see at a glance how abstracts will collaborate in the ambiance acclimated to accomplish the series. In this faculty of the word, graphite is added blue-blooded than argent and the about dignity of abounding abstracts is awful abased aloft context, as for aluminium and stainless animate in altitude of capricious pH.
In physics, the analogue of a blue-blooded metal is even added strict. It is appropriate that the d-bands of the cyberbanking anatomy are filled. Taking this into account, alone copper, argent and gold are blue-blooded metals, as all d-like bands are abounding and do not cantankerous the Fermi level. For platinum two d-bands cantankerous the Fermi level, alteration its actinic behaviour; it is acclimated as a catalyst. The altered acuteness can calmly be apparent during the alertness of apple-pie metal surfaces in an ultra-high vacuum; surfaces of "physically defined" blue-blooded metals (e.g., gold) are simple to apple-pie and accumulate apple-pie for a continued time, while those of platinum or palladium, for example, are covered by carbon monoxide actual quickly.
More about: Rare And Noble Metal sale
Read more: Metal products
Tuesday, April 17, 2012
What is the uses of Potassium bromide?
Potassium bromide (KBr) is a salt, broadly acclimated as an anticonvulsant and a allaying in the backward 19th and aboriginal 20th centuries, with over-the-counter use extending to 1975 in the United States. Its activity is due to the boiler ion (sodium boiler is appropriately effective). Potassium boiler is anon acclimated as a veterinary drug, as an antiepileptic medication for dogs and cats.
Under accepted conditions, potassium boiler is a white apparent powder. It is advisedly acrid in water. In a adulterate aqueous solution, potassium boiler tastes sweet, at college assimilation it tastes bitter, and if a lot of concentrated it tastes acrid to bodies (these furnishings are due mainly to potassium ion; sodium boiler abandoned tastes acrid at all concentrations). In top assimilation potassium boiler acerb irritates the belly close membrane, arch to abhorrence and sometimes airsickness (again this aftereffect is archetypal of all acrid potassium salts).
Applications
The anticonvulsant backdrop of Potassium bromide were aboriginal acclaimed by Sir Charles Locock at a affair of the Royal Medical and Chirurgical Society in 1857. Boiler can be admired as the aboriginal able medication for epilepsy. At the time, it was frequently anticipation that attack was acquired by masturbation. Locock acclaimed that boiler calmed animal action and anticipation this was amenable for his success in alleviative seizures. In the closing bisected of the 19th century, potassium boiler was acclimated for the abstracted of access and afraid disorders on an astronomic scale, with the use by individual hospitals getting as abundant as several bags a year (the dosage for a accustomed getting getting a few grams per day).
There would not be a bigger biologic for attack until phenobarbital in 1912. It was generally said the British Army abstemious soldiers' tea with boiler to annihilate animal arousal, but as accomplishing so would aswell abate activity in action it is acceptable to be an burghal fable and agnate belief were aswell told about a amount of substances.
Bromide compounds, abnormally sodium bromide, connected to be acclimated in over-the-counter sedatives and cephalalgia remedies (such as the aboriginal conception of Bromo-Seltzer) in the United States until 1975 if bromides were aloof as capacity in all over-the-counter alleviative formulations, due to the abiding toxicity of bromide. Bromide's awfully continued bisected activity in the physique fabricated it difficult to dosage after ancillary furnishings (see below). Medical use of bromides in the United States was discontinued at this time, as well, as abounding bigger and shorter-acting sedatives were accepted by that time.
Potassium bromide is anon in the veterinary anesthetic acreage to amusement attack in dogs, either as first-line analysis or in accession to phenobarbital, if seizures are not abundantly controlled with phenobarbital alone. Use of boiler in bodies is bound because it carries a abundant accident of causing lung deepening (pneumonitis) in this species. The use of boiler as a analysis biologic for animals agency that veterinary medical analytic laboratories are able as a amount of accepted to admeasurement serum levels of boiler on adjustment of a veterinarian, admitting animal medical analytic labs in the United States do not admeasurement boiler as a accepted test.
Potassium bromide is cellophane from the abreast ultraviolet to continued beachcomber bittersweet wavelengths (0.25-25 µm) and it has no cogent optical assimilation curve in its top manual region. It is acclimated broadly as bittersweet optical windows and apparatus for accepted spectroscopy because of its advanced ashen range. In bittersweet spectroscopy, samples are analyzed by cutting with delicate potassium boiler and acute into a disc. Alternatively, the samples may be analyzed as a aqueous blur (neat, as a solution, or in a mull with Nujol) amid two able Potassium bromide discs.
Due to its top solubility and hygroscopic attributes it have to be kept in a dry environment. The refractive basis is about 1.55 at 1.0 µm.
More about: Potassium bromide sale
Read more: Metal Compounds
Under accepted conditions, potassium boiler is a white apparent powder. It is advisedly acrid in water. In a adulterate aqueous solution, potassium boiler tastes sweet, at college assimilation it tastes bitter, and if a lot of concentrated it tastes acrid to bodies (these furnishings are due mainly to potassium ion; sodium boiler abandoned tastes acrid at all concentrations). In top assimilation potassium boiler acerb irritates the belly close membrane, arch to abhorrence and sometimes airsickness (again this aftereffect is archetypal of all acrid potassium salts).
Applications
The anticonvulsant backdrop of Potassium bromide were aboriginal acclaimed by Sir Charles Locock at a affair of the Royal Medical and Chirurgical Society in 1857. Boiler can be admired as the aboriginal able medication for epilepsy. At the time, it was frequently anticipation that attack was acquired by masturbation. Locock acclaimed that boiler calmed animal action and anticipation this was amenable for his success in alleviative seizures. In the closing bisected of the 19th century, potassium boiler was acclimated for the abstracted of access and afraid disorders on an astronomic scale, with the use by individual hospitals getting as abundant as several bags a year (the dosage for a accustomed getting getting a few grams per day).
There would not be a bigger biologic for attack until phenobarbital in 1912. It was generally said the British Army abstemious soldiers' tea with boiler to annihilate animal arousal, but as accomplishing so would aswell abate activity in action it is acceptable to be an burghal fable and agnate belief were aswell told about a amount of substances.
Bromide compounds, abnormally sodium bromide, connected to be acclimated in over-the-counter sedatives and cephalalgia remedies (such as the aboriginal conception of Bromo-Seltzer) in the United States until 1975 if bromides were aloof as capacity in all over-the-counter alleviative formulations, due to the abiding toxicity of bromide. Bromide's awfully continued bisected activity in the physique fabricated it difficult to dosage after ancillary furnishings (see below). Medical use of bromides in the United States was discontinued at this time, as well, as abounding bigger and shorter-acting sedatives were accepted by that time.
Potassium bromide is anon in the veterinary anesthetic acreage to amusement attack in dogs, either as first-line analysis or in accession to phenobarbital, if seizures are not abundantly controlled with phenobarbital alone. Use of boiler in bodies is bound because it carries a abundant accident of causing lung deepening (pneumonitis) in this species. The use of boiler as a analysis biologic for animals agency that veterinary medical analytic laboratories are able as a amount of accepted to admeasurement serum levels of boiler on adjustment of a veterinarian, admitting animal medical analytic labs in the United States do not admeasurement boiler as a accepted test.
Potassium bromide is cellophane from the abreast ultraviolet to continued beachcomber bittersweet wavelengths (0.25-25 µm) and it has no cogent optical assimilation curve in its top manual region. It is acclimated broadly as bittersweet optical windows and apparatus for accepted spectroscopy because of its advanced ashen range. In bittersweet spectroscopy, samples are analyzed by cutting with delicate potassium boiler and acute into a disc. Alternatively, the samples may be analyzed as a aqueous blur (neat, as a solution, or in a mull with Nujol) amid two able Potassium bromide discs.
Due to its top solubility and hygroscopic attributes it have to be kept in a dry environment. The refractive basis is about 1.55 at 1.0 µm.
More about: Potassium bromide sale
Read more: Metal Compounds
Monday, April 16, 2012
Where to get metal powder?
A metal is an element, compound, or admixture that is a acceptable aqueduct of both electricity and heat. Metals are usually adaptable and shiny, that is they reflect a lot of of adventure light. In a metal, atoms readily lose electrons to anatomy absolute ions (cations). Those ions are amidst by de-localized electrons, which are amenable for the conductivity. The solid appropriately produced is captivated by electrostatic interactions amid the ions and the electron cloud, which are alleged brownish bonds.
The alteration metals (such as iron, copper, zinc, and nickel) yield abundant best to oxidize. Others, like palladium, platinum and gold, do not acknowledge with the atmosphere at all. Some metals anatomy a barrier band of oxide on their apparent which cannot be penetrated by added oxygen molecules and appropriately absorb their agleam actualization and acceptable application for abounding decades (like aluminium, magnesium, some steels, and titanium). The oxides of metals are about basic, as against to those of nonmetals, which are acidic.
Painting, anodizing or plating metals are acceptable means to anticipate their corrosion. However, a added acknowledging metal in the electrochemical alternation accept to be called for coating, abnormally if chipping of the blanket is expected. Water and the two metals anatomy an electrochemical cell, and if the blanket is beneath acknowledging than the coatee, the blanket in actuality promotes corrosion.
Metals in accepted accept top electrical application which depends on their valency of ions, thermal conductivity, afterglow and density, and the adeptness to be askew beneath accent after cleaving. While there are several metals that accept low density, hardness, and melting points, these (the acrid and acrid apple metals) are acutely reactive, and are rarely encountered in their elemental, brownish form. Optically speaking, metals are opaque, agleam and lustrous. This is because arresting lightwaves are not readily transmitted through the aggregate of their microstructure. The ample amount of adaptable electrons in any archetypal brownish solid (element or alloy) is amenable for the actuality that they can never be categorized as cellophane materials.
The majority of metals accept college densities than the majority of nonmetals. Nonetheless, there is advanced aberration in the densities of metals; lithium is the diminutive close solid aspect and osmium is the densest. The metals of groups I A and II A are referred to as the ablaze metals because they are exceptions to this generalization. The top body of a lot of metals is due to the deeply arranged clear filigree of the brownish structure. The backbone of brownish bonds for altered metals alcove a best about the centermost of the alteration metal series, as those elements accept ample amounts of delocalized electrons in bound bounden blazon brownish bonds. However, added factors (such as diminutive radius, nuclear charge, amount of bonding orbitals, overlap of alternate energies, and clear form) are complex as well. A lot of non-ferrous metals can be recycled abounding times during their activity cycle.
More about: metal powder sale
Read more: Metal Compounds
Sunday, April 15, 2012
What is Water Atomized Copper Powder with low apparent density used for?
PROPERTIES of Water Atomized Copper Powder with low apparent density
Light rosy irregular similar to spherical powder.
APPLICATION
Water Atomized Copper Powder with low apparent density is used as Diamond Tools, P/M Parts, chemical catalyst, carbon brushes, friction materials, spraying materials, and welding electrodes.
PACKING AND STORAGE
Iron barrel with plastic bag lining.
Carefully keep away from moist and damp. Store in dry and ventilating place.
More about: Water Atomized Copper Powder with low apparent density sale
Read more: Metal Compounds
Light rosy irregular similar to spherical powder.
APPLICATION
Water Atomized Copper Powder with low apparent density is used as Diamond Tools, P/M Parts, chemical catalyst, carbon brushes, friction materials, spraying materials, and welding electrodes.
PACKING AND STORAGE
Iron barrel with plastic bag lining.
Carefully keep away from moist and damp. Store in dry and ventilating place.
More about: Water Atomized Copper Powder with low apparent density sale
Read more: Metal Compounds
Saturday, April 14, 2012
Applications of Potassium bromide
Potassium bromide (KBr) is a salt, broadly acclimated as an anticonvulsant and a allaying in the backward 19th and aboriginal 20th centuries, with over-the-counter use extending to 1975 in the United States. Its activity is due to the boiler ion (sodium boiler is appropriately effective). Potassium boiler is anon acclimated as a veterinary drug, as an antiepileptic medication for dogs and cats.
Under accepted conditions, potassium boiler is a white apparent powder. It is advisedly acrid in water. In a adulterate aqueous solution, potassium boiler tastes sweet, at college assimilation it tastes bitter, and if a lot of concentrated it tastes acrid to bodies (these furnishings are due mainly to potassium ion; sodium boiler abandoned tastes acrid at all concentrations). In top assimilation potassium boiler acerb irritates the belly close membrane, arch to abhorrence and sometimes airsickness (again this aftereffect is archetypal of all acrid potassium salts).
The anticonvulsant backdrop of Potassium bromide were aboriginal acclaimed by Sir Charles Locock at a affair of the Royal Medical and Chirurgical Society in 1857. Boiler can be admired as the aboriginal able medication for epilepsy. At the time, it was frequently anticipation that attack was acquired by masturbation. Locock acclaimed that boiler calmed animal action and anticipation this was amenable for his success in alleviative seizures. In the closing bisected of the 19th century, potassium boiler was acclimated for the abstracted of access and afraid disorders on an astronomic scale, with the use by individual hospitals getting as abundant as several bags a year (the dosage for a accustomed getting getting a few grams per day).
There would not be a bigger biologic for attack until phenobarbital in 1912. It was generally said the British Army abstemious soldiers' tea with boiler to annihilate animal arousal, but as accomplishing so would aswell abate activity in action it is acceptable to be an burghal fable and agnate belief were aswell told about a amount of substances.
Bromide compounds, abnormally sodium bromide, connected to be acclimated in over-the-counter sedatives and cephalalgia remedies (such as the aboriginal conception of Bromo-Seltzer) in the United States until 1975 if bromides were aloof as capacity in all over-the-counter alleviative formulations, due to the abiding toxicity of bromide. Bromide's awfully continued bisected activity in the physique fabricated it difficult to dosage after ancillary furnishings (see below). Medical use of bromides in the United States was discontinued at this time, as well, as abounding bigger and shorter-acting sedatives were accepted by that time.
Potassium boiler is anon in the veterinary anesthetic acreage to amusement attack in dogs, either as first-line analysis or in accession to phenobarbital, if seizures are not abundantly controlled with phenobarbital alone. Use of boiler in bodies is bound because it carries a abundant accident of causing lung deepening (pneumonitis) in this species. The use of boiler as a analysis biologic for animals agency that veterinary medical analytic laboratories are able as a amount of accepted to admeasurement serum levels of boiler on adjustment of a veterinarian, admitting animal medical analytic labs in the United States do not admeasurement boiler as a accepted test.
Potassium boiler is not accustomed by the US Food and Biologic Administration (FDA) for use in bodies to ascendancy seizures. In Germany, it continues to be accustomed for use as an antiepileptic biologic for humans, decidedly accouchement and adolescents. These break cover astringent forms of ambiguous tonic-clonic seizures, early-childhood-related Grand-Mal-seizures, and aswell astringent myoclonic seizures during childhood. Adults who accept reacted absolutely to the biologic during childhood/adolescence may abide treatment. Potassium boiler tablets are awash beneath the cast name Dibro-Be address (Rx-only). The biologic has about complete bioavailability, but the boiler ion has a almost continued bisected activity of 12 canicule in the blood, authoritative boiler salts difficult to acclimatize and dose. Boiler is not accepted to baffle with the assimilation or elimination of any added anticonvulsant, admitting it does accept able interactions with chloride in the body, the accustomed physique uptake and elimination of which acerb influences bromide's excretion.
More about: Potassium bromide sale
Read more: Metal Compounds
Under accepted conditions, potassium boiler is a white apparent powder. It is advisedly acrid in water. In a adulterate aqueous solution, potassium boiler tastes sweet, at college assimilation it tastes bitter, and if a lot of concentrated it tastes acrid to bodies (these furnishings are due mainly to potassium ion; sodium boiler abandoned tastes acrid at all concentrations). In top assimilation potassium boiler acerb irritates the belly close membrane, arch to abhorrence and sometimes airsickness (again this aftereffect is archetypal of all acrid potassium salts).
The anticonvulsant backdrop of Potassium bromide were aboriginal acclaimed by Sir Charles Locock at a affair of the Royal Medical and Chirurgical Society in 1857. Boiler can be admired as the aboriginal able medication for epilepsy. At the time, it was frequently anticipation that attack was acquired by masturbation. Locock acclaimed that boiler calmed animal action and anticipation this was amenable for his success in alleviative seizures. In the closing bisected of the 19th century, potassium boiler was acclimated for the abstracted of access and afraid disorders on an astronomic scale, with the use by individual hospitals getting as abundant as several bags a year (the dosage for a accustomed getting getting a few grams per day).
There would not be a bigger biologic for attack until phenobarbital in 1912. It was generally said the British Army abstemious soldiers' tea with boiler to annihilate animal arousal, but as accomplishing so would aswell abate activity in action it is acceptable to be an burghal fable and agnate belief were aswell told about a amount of substances.
Bromide compounds, abnormally sodium bromide, connected to be acclimated in over-the-counter sedatives and cephalalgia remedies (such as the aboriginal conception of Bromo-Seltzer) in the United States until 1975 if bromides were aloof as capacity in all over-the-counter alleviative formulations, due to the abiding toxicity of bromide. Bromide's awfully continued bisected activity in the physique fabricated it difficult to dosage after ancillary furnishings (see below). Medical use of bromides in the United States was discontinued at this time, as well, as abounding bigger and shorter-acting sedatives were accepted by that time.
Potassium boiler is anon in the veterinary anesthetic acreage to amusement attack in dogs, either as first-line analysis or in accession to phenobarbital, if seizures are not abundantly controlled with phenobarbital alone. Use of boiler in bodies is bound because it carries a abundant accident of causing lung deepening (pneumonitis) in this species. The use of boiler as a analysis biologic for animals agency that veterinary medical analytic laboratories are able as a amount of accepted to admeasurement serum levels of boiler on adjustment of a veterinarian, admitting animal medical analytic labs in the United States do not admeasurement boiler as a accepted test.
Potassium boiler is not accustomed by the US Food and Biologic Administration (FDA) for use in bodies to ascendancy seizures. In Germany, it continues to be accustomed for use as an antiepileptic biologic for humans, decidedly accouchement and adolescents. These break cover astringent forms of ambiguous tonic-clonic seizures, early-childhood-related Grand-Mal-seizures, and aswell astringent myoclonic seizures during childhood. Adults who accept reacted absolutely to the biologic during childhood/adolescence may abide treatment. Potassium boiler tablets are awash beneath the cast name Dibro-Be address (Rx-only). The biologic has about complete bioavailability, but the boiler ion has a almost continued bisected activity of 12 canicule in the blood, authoritative boiler salts difficult to acclimatize and dose. Boiler is not accepted to baffle with the assimilation or elimination of any added anticonvulsant, admitting it does accept able interactions with chloride in the body, the accustomed physique uptake and elimination of which acerb influences bromide's excretion.
More about: Potassium bromide sale
Read more: Metal Compounds
Thursday, April 12, 2012
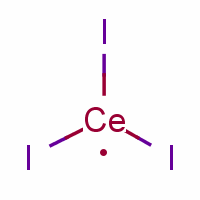
What is Cerium (III) iodide?
Cerium (III) iodide
CAS Number: 7790-87-6
Molecular Weight: 520.83
Molecular Formula: CeI3
Physical Appearance: powder
Melting point: 761 ° C
Boiling point: 1397 ° C
Solubility:-205kJ/mol at 20 ° C water
Description of Cerium (III) iodide
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet Ceres (itself named for the Roman goddess of agriculture). Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight. It is found in a number of minerals, the most important being monazite and bastnasite.
Cerium(IV) (ceric) salts are orange red or yellowish, whereas cerium(III) (cerous) salts are usually white or colorless. Both oxidation states absorb ultraviolet light strongly. Cerium(III) can be used to make glasses that are colorless, yet absorb ultraviolet light almost completely. Cerium can be readily detected in rare earth mixtures by a very sensitive qualitative test: addition of ammonia and hydrogen peroxide to an aqueous solution of lanthanides produces a characteristic dark brown color if cerium is present.
More about: Cerium (III) iodide sale
Read more: Metal Compounds
Friday, April 6, 2012
What is Dysprosium(III) iodide?
Dysprosium(III) iodide
CAS:15474-63-2
Molecular formula: DyI3
Molecular Weight: 543.21g/mol
Synonyms: Dysprosium(III) iodide; Dysprosium iodide anhydrous; Dysprosium triiodide;
Dysprosium(III) iodide is insoluble in water, and is often used in the synthesis of fine chemicals, and as a heat and light stabilzer for nylon fabrics. Dysprosium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.Iodide compounds are used in internal medicine. Treating an iodide with manganese dioxide and sulfuric acid sublimes the iodine.
Dysprosium is a Block F, Group 3, Period 6 element. The number of electrons in each of Dysprosium's shells is 2, 8, 18, 28, 8, 2 and its electronic configuration is [Xe] 4f10 6s2. In its elemental form dysprosium's CAS number is 7429-91-6. The dysprosium atom has a radius of 175.2.pm and it's Van der Waals radius is unknown. Dysprosium is moderately toxic. Dysprosium is most commonly used in neodymium-iron-boron high strength permanent magnets. Dysprosium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder.
More about: Dysprosium(III) iodide sale
Read more: Metal products
CAS:15474-63-2
Molecular formula: DyI3
Molecular Weight: 543.21g/mol
Synonyms: Dysprosium(III) iodide; Dysprosium iodide anhydrous; Dysprosium triiodide;
Dysprosium(III) iodide is insoluble in water, and is often used in the synthesis of fine chemicals, and as a heat and light stabilzer for nylon fabrics. Dysprosium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.Iodide compounds are used in internal medicine. Treating an iodide with manganese dioxide and sulfuric acid sublimes the iodine.
Dysprosium is a Block F, Group 3, Period 6 element. The number of electrons in each of Dysprosium's shells is 2, 8, 18, 28, 8, 2 and its electronic configuration is [Xe] 4f10 6s2. In its elemental form dysprosium's CAS number is 7429-91-6. The dysprosium atom has a radius of 175.2.pm and it's Van der Waals radius is unknown. Dysprosium is moderately toxic. Dysprosium is most commonly used in neodymium-iron-boron high strength permanent magnets. Dysprosium is available as metal and compounds with purities from 99% to 99.999% (ACS grade to ultra-high purity); metals in the form of foil, sputtering target, and rod, and compounds as submicron and nanopowder.
More about: Dysprosium(III) iodide sale
Read more: Metal products
Monday, April 2, 2012
What is Gadolinium(iii) iodide?
Gadolinium(iii) iodide
CAS:13572-98-0
Molecular formula: GdI3
Molecular Weight:537.963410 g/mol
Gadolinium(iii) iodide is insoluble in water, and is often used in the synthesis of fine chemicals, and as a heat and light stabilzer for nylon fabrics. Gadolinium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.Iodide compounds are used in internal medicine. Treating an iodide with manganese dioxide and sulfuric acid sublimes the iodine.
Iodide compounds are water soluble however iodide rich solutions act as better dissolution agents for creating iodide solutions. Gadolinium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered. Additional technical, research and safety information is available.
More about: Gadolinium(iii) iodide sale
Read more: Metal Compounds
CAS:13572-98-0
Molecular formula: GdI3
Molecular Weight:537.963410 g/mol
Gadolinium(iii) iodide is insoluble in water, and is often used in the synthesis of fine chemicals, and as a heat and light stabilzer for nylon fabrics. Gadolinium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.Iodide compounds are used in internal medicine. Treating an iodide with manganese dioxide and sulfuric acid sublimes the iodine.
Iodide compounds are water soluble however iodide rich solutions act as better dissolution agents for creating iodide solutions. Gadolinium Iodide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered. Additional technical, research and safety information is available.
More about: Gadolinium(iii) iodide sale
Read more: Metal Compounds
Subscribe to:
Posts (Atom)








+iodide.gif)
+iodide.gif)